|
Introduction
During endocytosis, cells need to be remodeled to permit the insertion of an antigen (bacteria or virus for example). For this purpose, the organism uses ESCRT machinery (Endosomal Sorting Complexes required to Transport) composed of four protein complexes: ESCRT0, I, II, III, which have different roles, from the recognition of proteins to their transport to the proteasome by ubiquitinylation. But this mechanism is not well known. That is why, I worked on the STAM2 protein, a part of ESCRT0, used for the linkage between ubiquitin and proteins; more precisely to confirm the pre-established UIM domain structure of STAM2 by Nuclear Magnetic Resonance (NMR) and Circular Dichroism (CD) analysis of UIM-SH3 protein (109 residues). I expose the CD here, a method based on the deviation of the light polarization plane.
Experimental conditions
To produce and analyze UIM domain of STAM2 with NMR and CD, E.Coli Gold were grown in LB medium (Luria Bertani) first and M9 medium next (M9 medium contains kanamycin, which is the specific antibiotic, and it was supplemented with 15N NH4Cl and 13C glucose as unique nitrogen and carbon sources). Bacteria can grow on this medium because they express the plasmid genes encoding resistance to kanamycin (an antibiotic) and production of UIM-SH3 protein linked to a NusA-6His label. The Optical Density (OD) at 280 nm was measured and after it rose to 0.6 UA, IPTG was added to force bacteria to produce the targeted protein. When the production was over, UIM-SH3 protein had been purified with two nickel affinity chromatographies and a gel filtration (between the two chromatographies, TEV was added, to cut the label linked to protein). After that, the OD at 200 nm of the protein was checked and the sample was diluted to obtain an OD lower than 1.5 UA (an OD of 1.37UA was obtained which corresponds to a concentration of 3.33 ÁM). Then, the CD analysis was performed at the Light Matter Institute lab with the following parameters: averaging on the five CD spectra saved from 205 to 256.6 nm with an integration time of 2.1 seconds, the CD was expressed in degrees with a sliding average of 10+10 neighbors.
Results
To process CD results, an Excel File was used to calculate the composition of secondary structures in the targeted protein from the concentration, the CD values, the wavelength, the cell path (1 cm here) and the number of residues. Then, my data were fitted by the program to extract the proportion of each kind of secondary structures elements (helix, beta-strand or random coil). The experimental and fitted curves are presented in the graph; and the proportion of secondary structures is given in the table, compared with the expected theoretical proportion with an uncertainty of one residue (representing a variation of 0.9%). 13% of the structure was in alpha helix in the experimental results, contrary to 14-15% in the theoretical one. However, only UIM can present an alpha helix because the SH3 domain is composed of five beta strands with a 3-10 helix. So, the CD analysis shows that the UIM domain of STAM2 is an alpha-helical structure, to the nearest residue.
Conclusion
The circular dichroism analysis of the UIM-SH3 domains of STAM2 confirmed the hypothesized UIM structure, i.e. an alpha-helical structure. However, there is a difference between experimental and theoretical results: it may be due to the statistics tool uncertainty of one residue or it is the capacity of the alpha helix structure to unfold, which corresponds to the UIM-SH3 dynamical structure results: UIM is dynamic, but its secondary structures seem to be stabilized when it binds to ubiquitin. Conversely, the structures of the other domains, VHS and SH3, present no change when they bind to ubiquitin. So, we have to wait for the NMR analysis results to confirm one of these hypotheses.
|
|
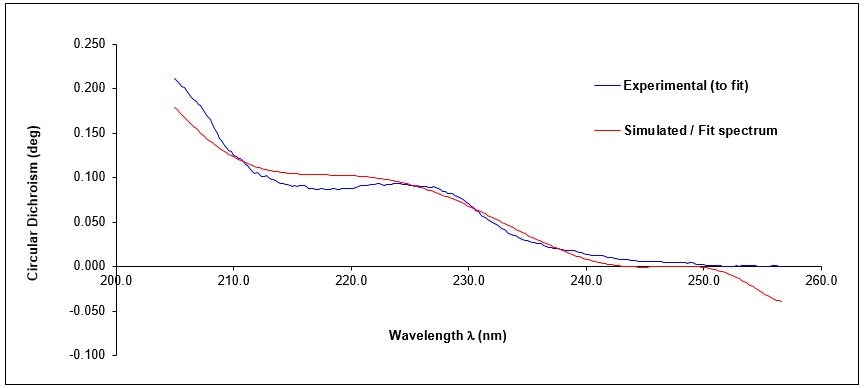
Circular dichroism curves as a function of wavelength, obtained by analysis of the UIM-SH3 domain of STAM2, in concentration of 3.33 ÁM at 25 ░ C.
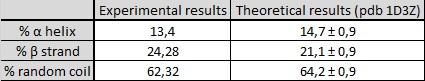
Table of results on the secondary structure composition of UIM-SH3 protein.
|




