|
Introduction
When farmers apply pesticides on their soils, a part is adsorbed into the soil, and another is routed to rivers, possibly leading to their contamination. The adsorption phenomena are very complex since a lot of chemical and physical parameters have to be considered. Indeed, an equilibrium concentration was established between the solid and the aqueous phase when pesticides are introduced into soil and water mixture. Adsorption observed in vineyard soils was simulated in a laboratory by reproducing similar conditions thanks to batch experiments. The purpose of this study was to better understand pesticide behaviors depending on soils characteristics (organic matter, clay and sand content).
Experimental conditions
Firstly, three vineyard soil horizons were collected at different depths. Then, seven pesticides were chosen to elaborate the assay. They are from different categories (herbicide, insecticide, fungicide) and have different structural and chemical compositions. Batch experiments were used to determine sorption isotherms and their corresponding Kd (sorption coefficient) values. To carry out the experiment, a sensitive analytical method UHPLC MS/MS (Ultra High Performance Liquid Chromatography coupled with tandem Mass Spectrometry) was used.
Quantification was achieved by Multiple Reaction Monitoring method and two fragmentation transitions were selected for each pesticide. MRM was used to be very selective. Analytical accuracy was enhanced using deuterated standard for internal calibration. The adsorption isotherms were carried out at five levels of pesticide concentrations to evaluate their influence on sorption. The experimental methodology consists in spiking a mixture of soil and water with a known concentration in pesticides (Figure 1). For the less contaminated samples a first step of sample pre-treatment by a solid phase extraction (SPE) was performed.
Results
The results suggest an established link between pesticides mass in solid phase and into aqueous phase (Figure 2). The chosen regression is a linear function and the slope is directly proportional to the Kd value. Consequently, adsorption is illustrated by the slope. Adsorption isotherms highlighted different pesticide behaviors. Firstly, most hydrophobic molecules (tebuconazole, linuron, chlorpiryphos-methyl) were mainly adsorbed on rich organic matter soils (Figure 2).
Then, one fungicide (azoxystrobin) was more retained on the clay-rich horizon. Indeed, this pesticide was probably ionized into the soil, then interactions which occurred cannot be known. One substance (chlortoluron) seems not to be influenced by soil composition; the isotherm slope was similar in each soil horizon (Figure 2). This is probably due to pesticide polarity which involves high solubility in water and thus, a reduced soil retention capacity. The fourth behavior type shows a difference between the three soils although no link with organic matter was established (dimethomorph and spiroxamin).
Conclusion
Even if Kd measures contain a lot of uncertainties due to the experimental and analytical steps, we are able to use the date and give an interpretation. This experimental study shows that pesticide adsorption depends on different parameters:
- pesticide physical-chemicals properties (solubility, polarity, structure)
- soil characteristics (clay and organic matter contents, pH)
|
|
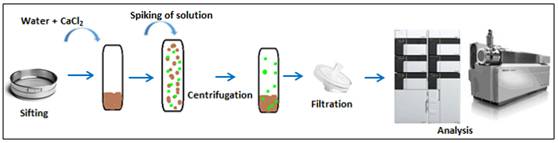
Different steps of the experiment
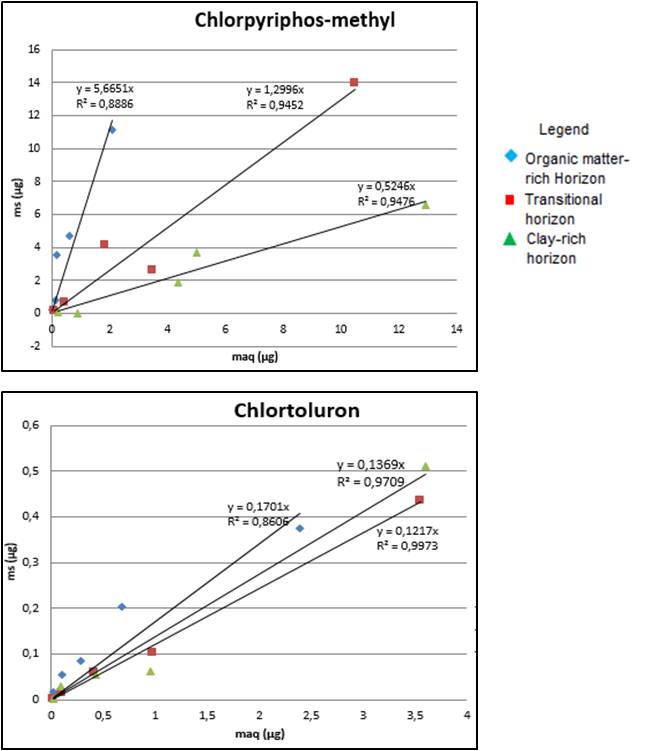
Adsorption isotherms of chlorpiryphos-methyl and chlortoluron
|




