|
Introduction
Polyamide 6.6, also called Nylon, is made from a nylon salt by a polycondensation reaction between adipic acid and hexamethylene diamine (Figure 1). The polyamide molecules have acidic and basic free ends. Their dosage permits to control the tincture of the final product. The results in concentrations of the terminal groups NH2 and COOH allow to determine the quality of the product but also to inform the customers who will achieve subsequently, the dye of the fibre.
The aim of the study is to show the optimization of the measurement quality and the safety of a potentiometric dosage method of polyamide by comparing two methods of dosage.
Experimental conditions
The potentiometric dosage of the polyamide was automated. It was necessary to measure two blanks, one control sample and then the samples of the production. The polyamide fibre (about 1.5 g) was
solubilized in 30 ml of a trifluoroethanol (TFE) / chloroform solution and then 4 mL of hydroxide tretrabutylammonium were added. The titratrating solution was a 0.1 M hydrochloric acid solution.
The potential was measured by a combined electrode comprising a glass electrode and a reference electrode. The measuring electrode was a glass membrane sensitive to protic concentration and the reference electrode was an Ag / AgCl electrode.
The final result in concentration of the terminal groups was given by a calculation between the equivalence volumes of the blanks and the samples. The results were in meq / kg, that means mmoles of the amine or carboxylic function per mass of sample.
Results
To improve the quality of the assay, a new method of analysis was set up. The old method used a solution of TFE / chloroform prepared by the technicians (with a high variability during the preparation). The new method was carried out with a solution of TFE / chloroform already prepared by a supplier. A comparison of the two methods was performed by studying the variability of blank volumes and the control samples through a statistical study. The new method of analysis showed a lower variability of results.
Moreover, the TFE / chloroform solvent was carcinogenic, mutagenic and toxic for reproduction (CMR) and required considerable care for its use. The safety of the workstation has been optimized with the new method. On the one hand, solvent preparation needed less manipulation. On the other hand, polypropylene cover was used for sample preparation instead of parafilm which disrupted the dosage.
Lastly, control charts for the concentration of NH2 terminal groups (Figure 2) and COOH were set up to improve the quality control of the analysis.
Conclusion
Thus the quality of the dosage has been improved. With the new method using a TFE / chloroform solution already prepared by the supplier, the variability of the results was lower and the reliability has been enhanced.
The dosage safety of the polyamide end groups has been updated by reducing the manipulation of the unsafe solvent and the adaptation of the workstation.
|
|
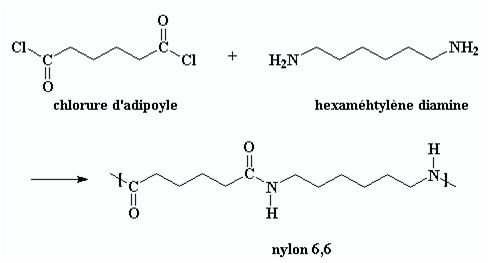
Figure 1 : Synthesis reaction of polyamide 6,6
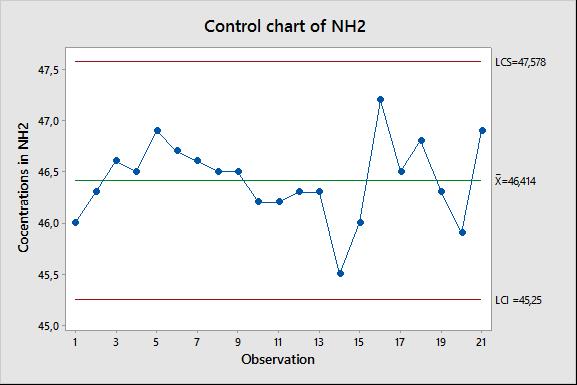
Figure 2 : Control chart of NH2 concentration of the control sample
|




