|
Introduction
Vitamin D is a soluble vitamin in fat which permits to determinate bone metabolism in human body. It’s synthesized when the dermis’ cholesterol is affected by UltraViolet. Physicians agree to a ratio of 30 to 75 ng/mL of vitamin D in blood. A deficiency can lead to rickets for children or osteoporosis for seniors. Reaction of quantitative analysis of vitamin D is based on immunoassay principle with revelation by electrochemiluminescence.
As part of medical laboratory accreditation, an examination of the quantitative analysis method is necessary. For that purpose, we used a method elaborated by Roche Company on Cobas 6000. We had to compare repeatability and reproducibility with Roche Cie and study the main influence of this quantitative analysis, the haemolysis.
Experimental conditions
The laboratory used reagent Vitamin D from Roche Cie. This reagent pack includes different compartments which contain chemical elements to produce an electrochemiluminescence reaction : vitamin D (25-OH)-biotin complex, streptavidin protein-magnetic micro particles complex and vitamin D binding protein-ruthenium complex. I evaluated repeatability and reproducibility with PreciControl Varia 1 and 2 control sample which contains vitamin D (25-OH). For each control the Company supplied a concentration level and a standard deviation respectively 19,5 ± 2,1 ng/mL and 33,6 ± 3,4 ng/mL.
The laboratory used two levels of concentration of Vitamin D calibration samples from Roche Cie : 2 and 45 ng/mL.
The principle of quantitative analysis of vitamin D is based on immunoassay with revelation by electrochemiluminescence between a platinum electrode and reference electrode.
Repeatability is assessed on 30 passages of each PreciControl Varia accomplished the same day and reproducibility is assessed during several days. We obtained the average values and the coefficient of variation for each level. We have to compare those values with those obtained with Roche Company.
To study haemolysis’ influence on quantitative analysis, I prepared hematite’s pool with 10x0,2 mL of blood from different patients. After a 1:3 dilution, I had a tube at 9,00 g/dL of haemolysis. Furthermore, I prepared a serum’s pool with 7x1 mL of serum from patients between 17 to 35 years old for vitamin D dosage.
Results
For repeatability, after 30 passages of each PreciControl Varia 1 and 2, we noticed that coefficients of variation were under coefficients of variation of reproducibility. Then, we confirmed the repeatability of this quantitative analysis method for Vitamin D.
For reproducibility, after 30 passages of each PreciControl Varia level, we noticed that we had problems. Indeed, our average value was low compared to Roche Cie value. However, our coefficient of variation was right so we had an accuracy problem.
We called Roche Cie and we found out that Vitamin D calibration samples needed to be reconstituted each time a new reagent pack was loaded into the Cobas 6000 and had to be frozen for other calibrations. After this advice, we started a new reproducibility which is nearer to the average of Roche.
Influence of haemolysis’ study allows to determine a threshold under which haemolysis doesn’t have any influence on quantitative analysis of vitamin D. Roche Cie sets this limit at 124 µmol/L of free haemoglobin in blood but my research demonstrated that it’s at 242 µmol/L. So, I demonstrated that this influence exists and that we have to take it into account during future vitamin D analysis.
Conclusion
In the end, I have confirmed repeatability of the vitamin D quantitative analysis method but reproducibility is being reviewed with new use of Vitamin D calibration and reagent pack.
Furthermore, I demonstrated the influence of haemolysis above around 200 µmol/L of free haemoglobin in blood.
|
|
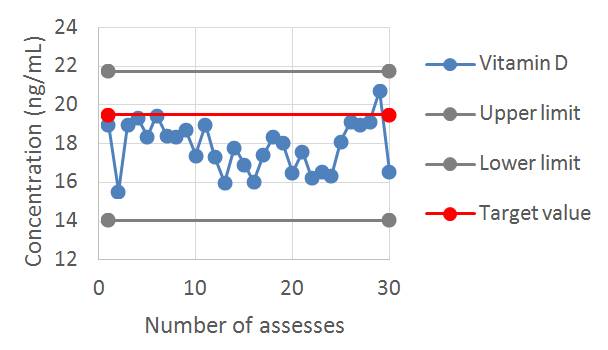
Reproducibility of PreciControl Varia Level 1. We observed all values were under the target value but all were between limits.
|



