|
Introduction
Lasso peptides constitute an interesting and original class of bacterial peptides. They are composed of a macrolactam cycle in which the C-terminal tail is trapped by cumbersome residues or disulfide bridges. The lasso peptides structure is all the more interesting that lasso peptides do not have all the same spectroscopic signature.
All lasso peptides have this interlaced structure but they differ by the presence or absence of disulfide bridges. So they can be ordered according to three types: type 1 which has two disulfide bridges, type 2 with no disulfide bridge and then type 3 with only one disulfide bridge.
There are several spectroscopic methods to analyze the lasso peptides structure like NMR, mass spectrometry and of course circular dichroism. The circular dichroism is a non-destructive spectroscopic method. The chiral sample is passed through by a beam of polarized light. It unequally absorbs the right and left circularly polarized light. The signal obtained gives the difference between both components according to the wavelength. In case of proteins and peptides, the circular dichroism gives some information on their global conformation. The percentage of the helical, sheet and random structures can therefore be determined.
In view of their original structure, the analysis of lasso peptides by circular dichroism is interesting to determine their spectroscopic signature because their CD spectra are different from the helical, sheet and random structures.
Experimental conditions
Different lasso peptides of type 2 (Mcc J25 and Capistruin) and 3 (BI-32169) were analyzed by circular dichroism at synchrotron SOLEIL, using a 0.02 cm pathlength cistern. The lasso peptides were taken into phosphate buffer at 10 mM and pH 7. The experience was realized at 25°C.
Then, a denaturation was performed at 90°C for 3 hours on the lasso peptide Rubrivinodin.
Results
The three CD spectra do not have the same appearance. They differ by the presence or not of the 230 nm band. In view of their amino acid sequence, the aromatic amino acids like tryptophan (W), tyrosine (Y) and phenylalanine (F) may be involved on this 230 nm band.
On the other hand, the spectra appearance of the Rubrivinodin changes over time so there is a temperature effect which causes the loss of the lasso structure.
Conclusion
The circular dichroism enables to characterize the spectroscopic signature of lasso peptides. It gives some information about the presence of the 230 nm band which is caused by the interaction between several aromatic amino acids. Moreover, the kinetic of the lasso structure loss can be followed by circular dichroism.
|
|
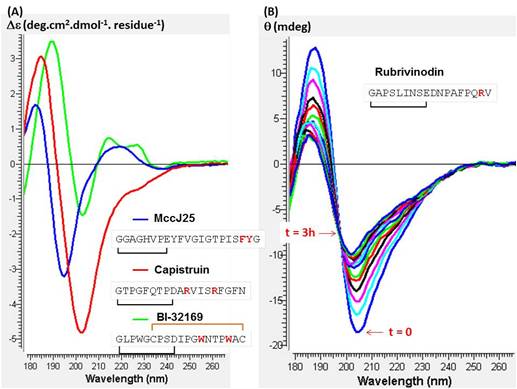
CD spectra of Mcc J25 (blue), Capistruin (red) and BI-32169 (green) at 25°C (A) and the denaturation of Rubrivinodin at 90°C for 3 hours (B) ; 0.02 cm pathlenght cistern ; phosphate buffer 10 mM pH7
|



