|
Introduction
Nowadays, sales and consumption of generic drugs are increasing due to the ageing of the population and the necessity for people and institutions to save money. MAP France is a pharmaceutical laboratory which works on generic drugs a lot. It does validations of analytical methods, pharmaceutical development and physico-chemical analysis.
The validation of analysis methods for the dosage of drugs or impurities is mandatory to obtain an Authorization of Launch on the Market (ALM) which allows to sell a drug, here Chondroïtin sulfate’s generic drug. Indeed, Chondoïtin sulfate is used to treat osteoarthritis and joint’s pain.
The aim of this validation method is to determine and verify the following parameters: linearity, accuracy, repeatability, reproducibility and ruggedness.
Experimental conditions
For this study, an anionic column is used: the 4.6*150 mm Waters, IC Pack™ Anion on a HPLC with a DAD detector (wavelength: 215 nm). The flow is 1 mL/min, the injected volume is 20 µL and the concentration injected is 5mg/mL in Chondroïtine (in water). Mobile phase (A) contains sodium chlorite (NaCl) at 2,5 M (146,1 g/L) and trishydroxyméthylaminométhane (TRIS) at 20 mM (2,42 g/L). Mobile phase A’s pH is adjusted to 3.00 with phosphoric acid (H3PO4) and phase B is water.
Chondroïtin is analyzed with this range, see figure 1.
Results
To perform a validation, it’s necessary to repeat testing many times and to use statistic to validate results.
On the one hand, two linearities were made: in water and in the matrix (with benzyl alcohol). A comparison between the two, with a Student test, proved they were no differences. Hence, samples were prepared in water for the following analysis. Moreover accuracy was tested thanks to linearities’ results and prepared sample concentration. Using a Cochran test, we concluded that the experimental results and the expected value were proportional.
On the other hand, a sample of Chondroïtin was prepared three times by three technicians and during three days, they were injected six times each. Then, they were analyzed to assess reproducibility. Meanwhile, ten injections of another sample were analyzed to check repeatability. These two parameters were validated in regard to the coefficient of variation (CV), in the pharmaceutical field it must be less than 2%.
Finally, for the ruggedness four conditions were tested: +5% and -5% of TRIS and +5% and -5% of NaCl. Experimental results of these conditions were compared with normal conditions: areas of modified conditions’ peaks must be very close to areas of standard peaks.
Conclusion
The method was almost validated. Indeed, reproducibility and repeatability had a CV inferior to 2% (1.40% and 0.46% respectively). It means that this method can be used in other laboratories and during all the production and the marketing of drugs with Chondroïtin. However, the study of ruggedness didn’t allow saying that a tiny variation in TRIS or NaCl didn’t have any consequences on the analysis. It must be redone to make sure that these variations were not caused by sample preparation problems.
|
|
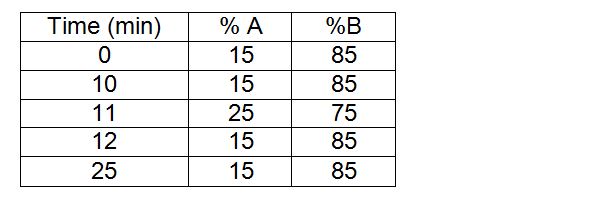
Elution gradient
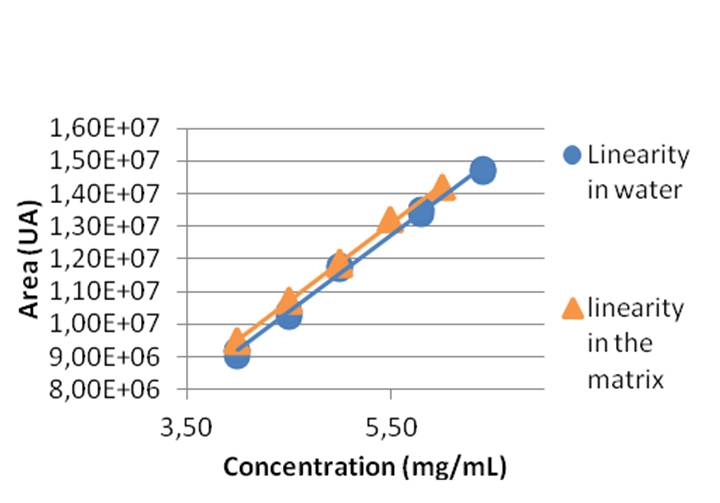
Comparison between lines of linearity in water and in the matrix
|




