|
Introduction
Ethanol is metabolized less than 0.1% in glucuronide or sulphate conjugates: ethyl glucuronide (EtG) and ethyl sulfate (EtS). These two ethanol metabolites are non-volatile soluble compounds eliminated in urine. Contrary to ethanol which remains detectable for 12 hours in saliva, breath, blood or urine, EtG and EtS can be detected in urine for 3-4 days. Furthermore, EtS, unlike EtG, remains stable in samples contaminated with bacteria. Thus, the determination of these two compounds in biological samples has a particular interest in predicting abstinence from ethanol in alcoholic patients. These two biomarkers can be useful in the procedure of recovering the driving license, in the therapeutic care of alcoholic patients but also to discriminate false-positive results in a post-mortem examination due to ethanol production by fermentation. The aim of this study was to validate a method of determination of EtG and EtS concentrations in urine samples by liquid chromatography coupled to tandem mass spectrometry (LC-MS²).
Experimental conditions
Material
The commercial MassChrom® kit, including an analytical column, mobile phases, calibrators, controls, internal standards, and all the reagents for sample preparation, dedicated to the determination of Ethyl Glucuronide and Ethyl Sulfate concentrations in urine by tandem mass spectrometry (LC-MS/MS) was purchased from Chromsystems (Chromsystems GmbH, Gräfelfing, Germany).
Analytical conditions
Analyses were performed in urine samples using a Shimadzu 20A UPLC system (Shimadzu, Marne la Vallée, France) coupled with a Sciex 3200 QTRAP tandem mass spectrometry (Life Sciences Holdings France SAS SCIEX, Villebon sur Yvette, France). UPLC conditions consisted in a gradient of mobile phases starting at a flow rate of 0.5 ml/min to finally reach a flow rate of 1.0 ml/min at 5.50 min. The total analysis time was 6.0 min. Multiple Reaction Monitoring (MRM) mode was used as the mode of data acquisition and two transitions were monitored for each component, one for quantifier ion and one for qualifier ion. The selected transitions were (221 → 75) and (221 → 85) for EtG and (125 → 80) and (125 → 97) for EtS for quantifier and qualifier ions, respectively. The selected transitions for the internal standards were (226 → 75) for EtG and (130 → 80) for EtS.
Sample Preparation
After a centrifugation at 9000g for 5 minutes, 50 µL of urine samples were diluted in 200 µL of internal standards solution. The mixture was mechanically vortexed for 10 sec and 200 µL of the mixture was transferred into an autosampler vial for UPLC. The same procedure was applied to the calibrators and controls preparation.
Method validation
The method was validated according to NF EN ISO 15189 and the COFRAC SH GTA 04 document entitled “Guide Technique d’accréditation de vérification (Portée A)/validation (Portée B) des méthodes en biologie médicale”. We used the criteria described for commercial methods validation (Portée A). This method validation includes usually the determination of 4 criteria: precision (repeatability and intermediate precision), accuracy, uncertainty and comparison with other methods.
The repeatability was calculated at three concentrations in ten replicates for each concentration (n=10) and the intermediate precision was determined over six days (n=6). Repeatability and intermediate precision were expressed in terms of coefficient of variation (CV) calculated as follow:
CV (%) = (standard deviation σ)/(mean µ) × 100
Precision assays were considered compliant if the percentage of CV was less than 20% as recommended by the French Society of Pharmacology and Therapeutics.
The cross-sample contamination was determined by measuring the concentrations of 3 controls of high levels (H1, H2, H3) followed by 3 controls of low levels (B1, B2, B3). This sequence was repeated 4 times (n=4). The percentage of cross-sample contamination was calculated as follow:
C = (mB1-mB3)/(mH-mB3) × 100
Results
The retention times were 2.55 min and 5.25 min for EtG and EtS, respectively. The calibration curves were linear between 73.5 to 11,900.0 µg/L for EtG and 39.2 to 3,060.0 µg/L for EtS. Limits of detection (LOD) and quantification (LOQ) were 24.4 µg/L and 64.4 µg/L for EtG and 15.5 µg/L and 43.6 µg/L for EtS, respectively.
All the tested criteria are presented in Table 1.
Conclusion
If precision, cross-sample contamination, LOD and LOQ were successfully validated according to NF EN ISO 15189, accuracy, uncertainty and comparison with other methods have to be performed. If the commercial Chromsystems method is a fast, simple and reproducible method, it presents the drawback to be a closed method where the composition of reagents and the nature of the analytical column are unknown, impeding its potential optimization. This method will be used routinely in the Department of Clinical Pharmacology and Toxicology for the monitoring of chronic alcoholic patients but also in the context of forensic analysis.
|
|
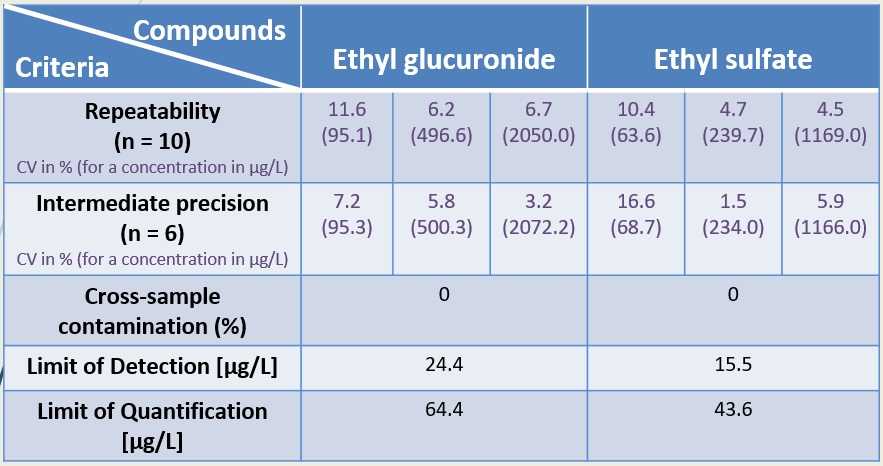
Table 1: Tested Criteria
|



