|
Introduction
Gamma-hydroxybutyrate (GHB) is an endogenous compound related to neuromodulator gamma-aminobutyric acid (GABA). First used in 1960 as medical anaesthetic, because of its exhilarating and amnesia inducing properties, GHB is henceforth used as a recreational drug of abuse and in drug-facilitated assaults. To discriminate endogenous GHB from exogenous intake, cut-off levels are used in forensic toxicology labs. The cut-off levels are 5 mg/L and 10 mg/L for the whole blood and urine from alive victims respectively. The aim of this study was to upgrade and validate a method to determine GHB levels in whole blood and urine samples in drug-facilitated assault fatalities by gas chromatography coupled to tandem mass spectrometry (GC-MS2).
Experimental conditions
Quantitation was performed using internal standardization. A six-point calibration curves from 1 to 50 mg/L were constructed with GHB spiked water solutions. Internal quality controls are used to assume calibration efficiency.
Before injection, sample preparation is required. 50 µL of sample and 20 µL of deutered internal standard (GHB-D6-Na) are diluted in acetonitrile (1/20) to induce protein precipitation and then decrease noise. To avoid GHB trapping in blood bottom, for whole blood samples only, dilution is split into two steps: the first one in water (1/4) and the second one in acetonitrile (1/5). After centrifugation, 100µL of surfactant is evaporated to allow silylation by 40 µL of BSTFA-1%TMS. For biological samples, a second preparation, based on a smaller aliquot, is performed to allow high level quantification (5µL (1/10) and 1µL (1/50) for blood and urine respectively).
Analysis is carried out with a Bruker 450 gas chromatograph in split mode coupled with a Bruker 320 Triple Quad Mass-Spectrometer, using Multiple Reaction Monitoring (MRM) mode. As illustrated in the following chromatogram (figure 1), five transitions are monitored: three for the GHB-diTMS (one quantifier 233.2→147 and two qualifiers 233.2→73 and 233.2→233.2) and two for the GHB-d6-diTMS (one quantifier 239.2→147 and one qualifier 239.2→73). Only one ion precursor was used because of urea-diTMS artefact. Urea is an endogenous compound, present in high levels in blood and urine. Unfortunately urea-diTMS has the same ionization fragments source than GHB and is coeluted with the used column. Because urea-diTMS weighs 204 g/mol, only heavier ions can be used as precursor ions.
GHB is identified by its retention time in regard to its internal standard one (7.95 min) and by the relative abundance of qualifiers transitions.
The method was validated by the determination of the accuracy profile using the e-noval V3.0 (Arlenda ®) software. For each type of biological preparation, the validation plan consisted in 3 series with their own calibration curves from the same set of GHB solutions and 4 to 6 levels of GHB spiked samples replicated 3 times. Total error was a priori fixed to 40% for a beta-risk of 10%. For diluted urine, this method can be less efficient (total error fixed to 50%) because high GHB urine levels have less toxicology relevance.
Results
Validation results are summarized in figure 2 (A) and illustrated by the accuracy profile obtained for not diluted urine showed in figure 2 (B).
Conclusion
The upgraded analytical method was successfully validated. To date, this method is used routinely in the Toxicology unit and will be submited to additional accreditation of the French Accreditation Committee (COFRAC). Next stage will be the development and validation of GHB dosage in hair.
|
|
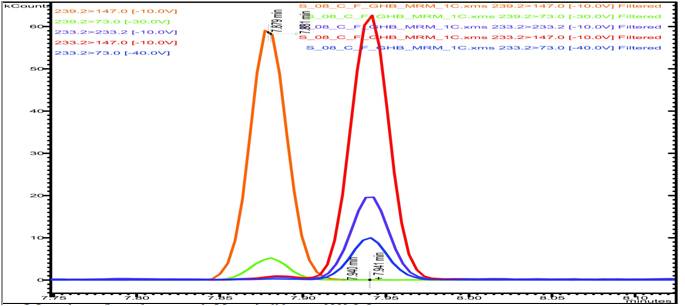
Chromatogram of a blood sample surcharged to 8 mg/L of GHB
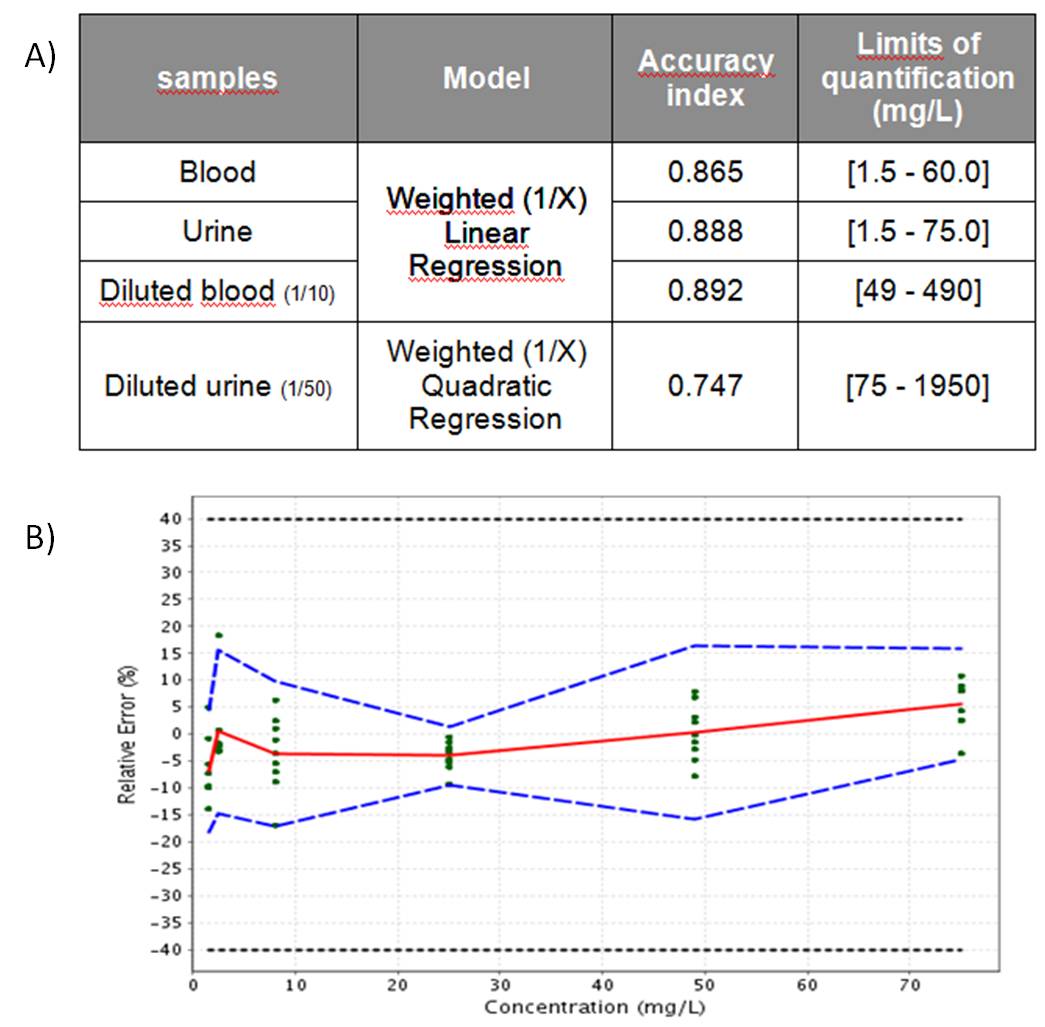
A) Validation results
B) Accuracy profile for not diluted urine
|




