|
Introduction
The first analysis in SCAN mode of each individual compound are being carried out, which makes it possible to determine the most intense precursor ions having a high m/z while paying attention to impurities such as silica (m/z = 207). Then the search for fragment ions with the Product Ion mode at different collision energies (CE) is performed. For this purpose, the precursor ion is broken to obtain fragments. Finally, the precursor ions/fragment ions transitions and the collision energies necessary for quantization in MRM mode are determined.
Experimental conditions
A sample containing a known amount of chlorophenols was injected into the GC: Agilent Technologies 7890A GC System. The GC part makes it possible to separate these compounds according to their affinities with the stationary phase and their boiling temperatures.
The different chromatograms obtained thus allow to define the method with the optimal gradient making it possible to separate all the chlorophenols. This gradient is described in the adjacent figure 1.
Following the separation, the chlorophenols were passed through the triple quadrupole: Agilent Technologies 7000 GC / MS Triple Quad.
First, compounds were analyzed in SCAN mode in order to determine the precursor ions. Then, with the same separation method, the chlorophenols were analyzed with the Product Ion mode, the m/z values of the precursor ions were parameterized. For various collision energy (CE) values, the fragment ions obtained with the greatest abundance were selected to determine the precursor ions/fragment ions necessary for quantization in MRM mode.
By fixing pairs of characteristic ions of a compound, the MS/MS detector is able to isolate it in a complex matrix and quantify it.
Those transitions determined and collected using the SCAN mode and the Product-Ion mode allows characterization and realization of a calibration line for each of the chlorophenols. Some concentration levels were chosen: 10μg/L, 20μg/L, 50μg/L, 100μg/L, 200μg/L, 400μg/L.
Results
In the adjacent table, these transitions make it possible to characterize each of the chlorophenols, although their structure is similar. The follow-up of two transitions per molecule is necessary for quantification. The first transition allows to quantify the molecule and the second to confirm the presence of the desired molecule (to qualify). One of the calibration lines (Pentachlorophenol) is shown in FIG. 2: The line is performed in quadratic mode because the range is too large to be in the linearity domain. The limit of quantification is thus fixed at 10μg/ L.
Conclusion
The work carried out has led to the development of the GC-MS / MS method and the optimization of this method currently achieves the objectives set: The instrumental LQ is 10μg/L as for the GC-ECD. It is not possible to go lower as the sensitivity is not sufficient. The range of the calibration curve is fixed from 10μg/L to 400μg/L and provides quadratic calibration curves specific to each chlorophenol.
|
|
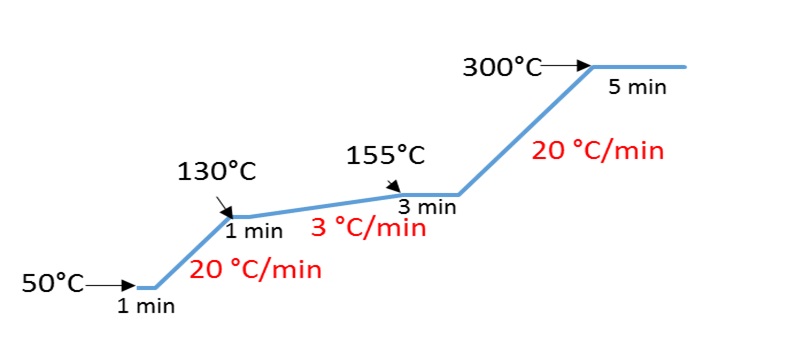
Figure 1: Optimal Gradient
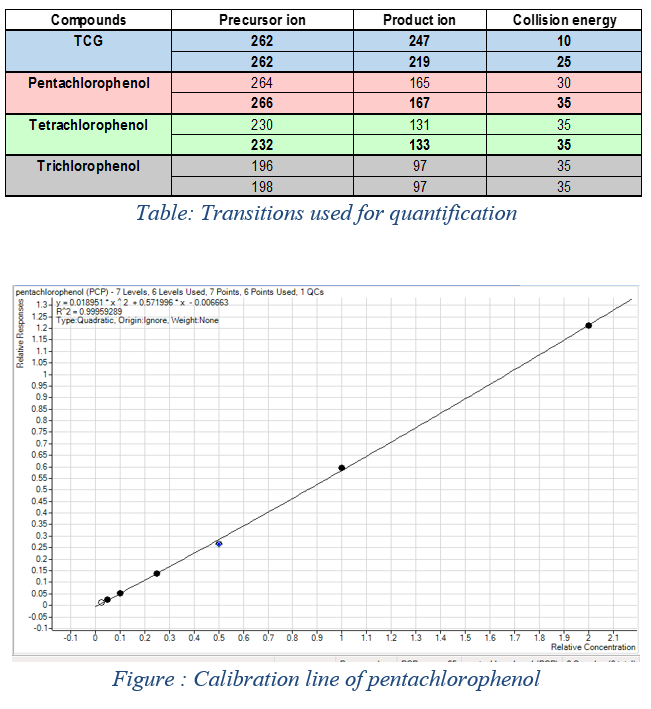
Figure 2: Calibration line of pentachlorophenol and transitions used
|




