|
Introduction
Tyrosinase is an enzyme involved in melanin biosynthesis. Melanin is found in mammals and plants to give the skin color. This biosynthesis pathway is based on the oxidation of L-DOPA to dopachrome by the tyrosinase. It consists in measuring the enzymatic activity by spectrophotometry. One unit of tyrosinase is defined as the amount of the enzyme required to oxidize 1 mol of L-DOPA per minute under the conditions chosen.
Experimental conditions
In this experiment, we added for the standard, 400 µL of enzyme tyrosinase and 550 µL of phosphate buffer which were incubated for 10minutes at 25°C. Then 50µL of L-DOPA substrate were added. First of all, the addition of the substrate started the reaction and then the absorbance was measured subsequently. In addition, the absorbance was recorded by spectrophotometry at 25°C every 30 seconds during 5 minutes in a black area. The activity of tyrosinase was monitored spectrophotometrically at 450nm by the dopachrome formation. In order to evaluate the accuracy and the precision of the methods, each reaction was replicated three times.
Results
The line graph showed the enzymatic tyrosinase absorbance according to the reaction time in seconds. The correlation coefficient was 0.9998 which was superior to 0.995. Accordingly, it is possible to conclude that the kinetics assay was linear between these two parameters. Moreover, the curve was linear because the reaction was happening at the initial velocity. The variation coefficient was less than 10% in each spot. Thus, this result showed the accuracy of the method.
Conclusion
To conclude, this enzymatic assay is used to measure the tyrosinase activity. This method could be used to evaluate the efficacy of some new tyrosinase inhibitors. These inhibitors might be interesting to prevent hyperpigmentation and browning vegetables.
|
|
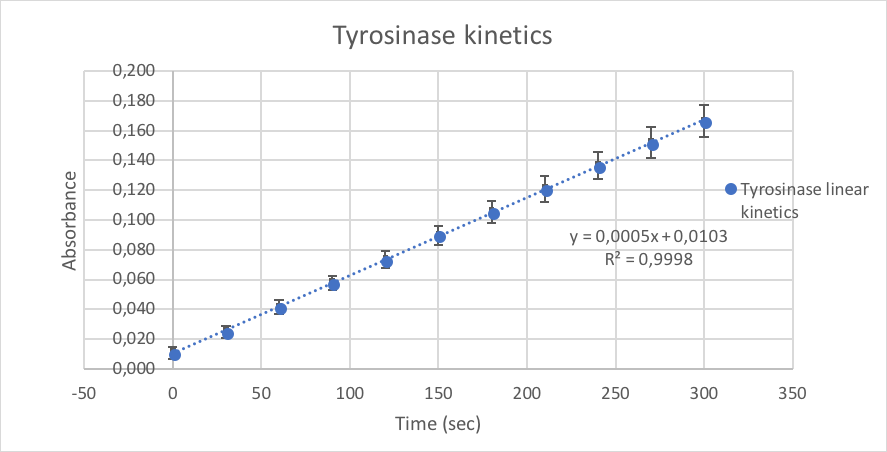
Line graphe of Tyrosinase kinetics showing the absorbance of product according to the reaction time at 450nm.
|



