|
Introduction
Medicines over 20 years old, patented by major pharmaceutical companies, can be copied by new laboratories. On average 30% less expensive, these copies called generic drugs are increasingly consumed. MAP France laboratory, specialized in the development of generics, examine dexamethasone sodium phosphate, an active substance used for its anti-inflammatory, immunosuppressive or anti-allergic effects.
The analysis of the molecule is carried out by High Performance Liquid Chromatography (HPLC) and based on the current ICH pharmaceutical standards. The development and validation of the analytical method allow to study the stability of dexamethasone sodium phosphate under denaturing conditions. This step is necessary to determine the preservation conditions of the drug to optimize its manufacturing and packaging process.
Experimental conditions
For the analysis of dexamethasone sodium phosphate on HPLC instrument, the selected parameters were based on those described by the European Pharmacopeia (EP) :
Column C8, 5µm, 4,6 x 250mm
Flow rate = 1mL/min, Temperature = 40°C.
Volume injection = 20µL
Samples concentration = 1mg/mL
Detection UV = 254nm
The separation was obtained with a gradient of two mobile phases prepared according to EP during 1h.
To run forced degradation assay, the active molecule was subjected to extreme conditions of temperature, UV radiations, pH (acid and basic) and oxidation.
Results
The specificity, linearity, repeatability, reproducibility, and the accuracy oh the method were validated. Stability of dexamethasone sodium phosphate was checked on 24 hours. After forced degradation assays, the substance active had to be recovered between 95% and 105%. After 48 hours, only pH basic and oxidation conditions could be validated (Figure 1). But, pH basic degraded the dexamethasone phosphate sodium in A impurity, by a hydrolysis (Figure 2).
Conclusion
The developed method for the dexamethasone sodium phosphate can be routinely used. Forced degradation tests finally indicated that the finished product have to be designed at neutral pH and kept away from light. However its preparation did not require an inert environment.
|
|
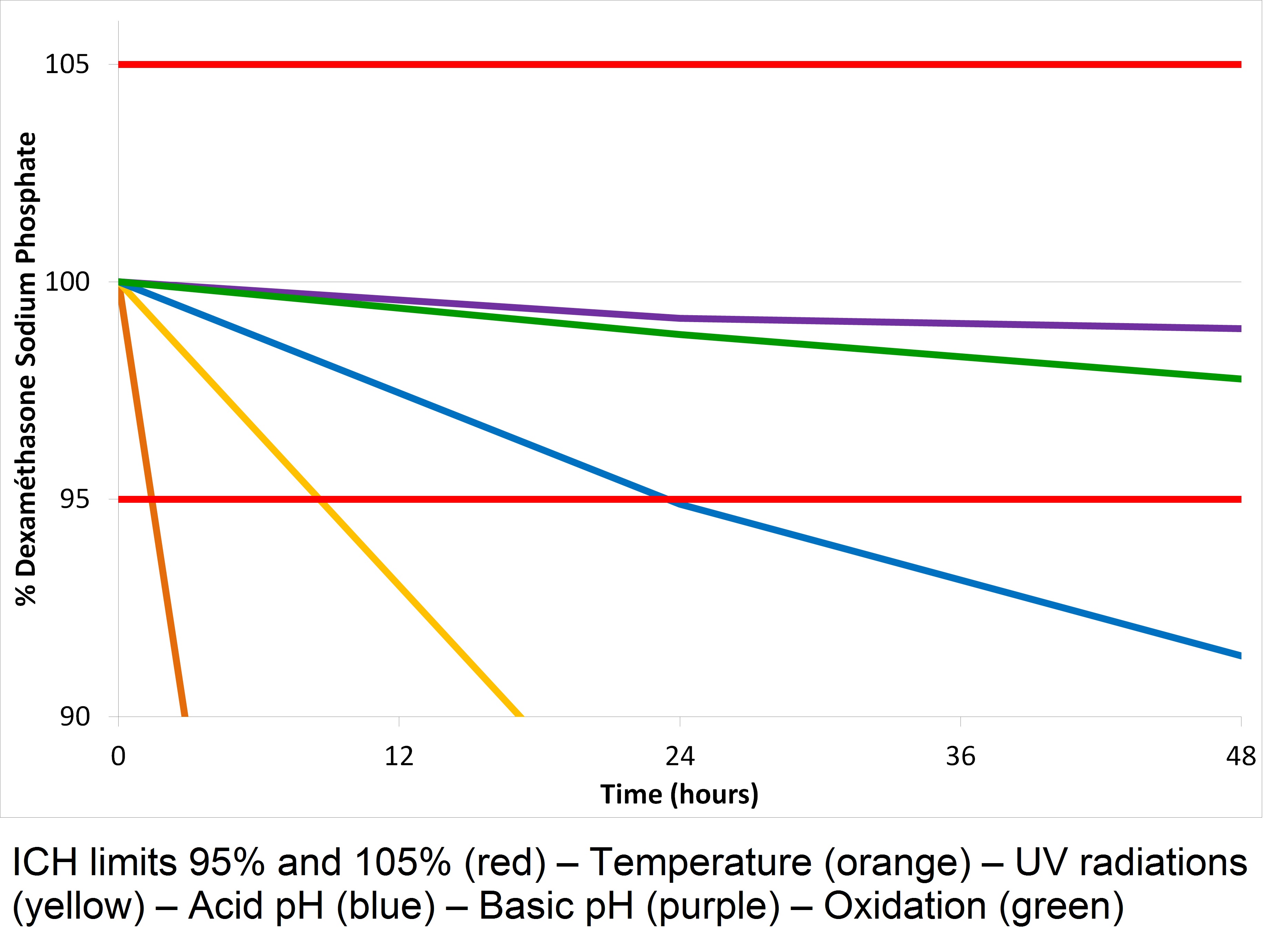
Percentage of dexamethasone sodium phosphate recovered over time in the various tests of forced degradation
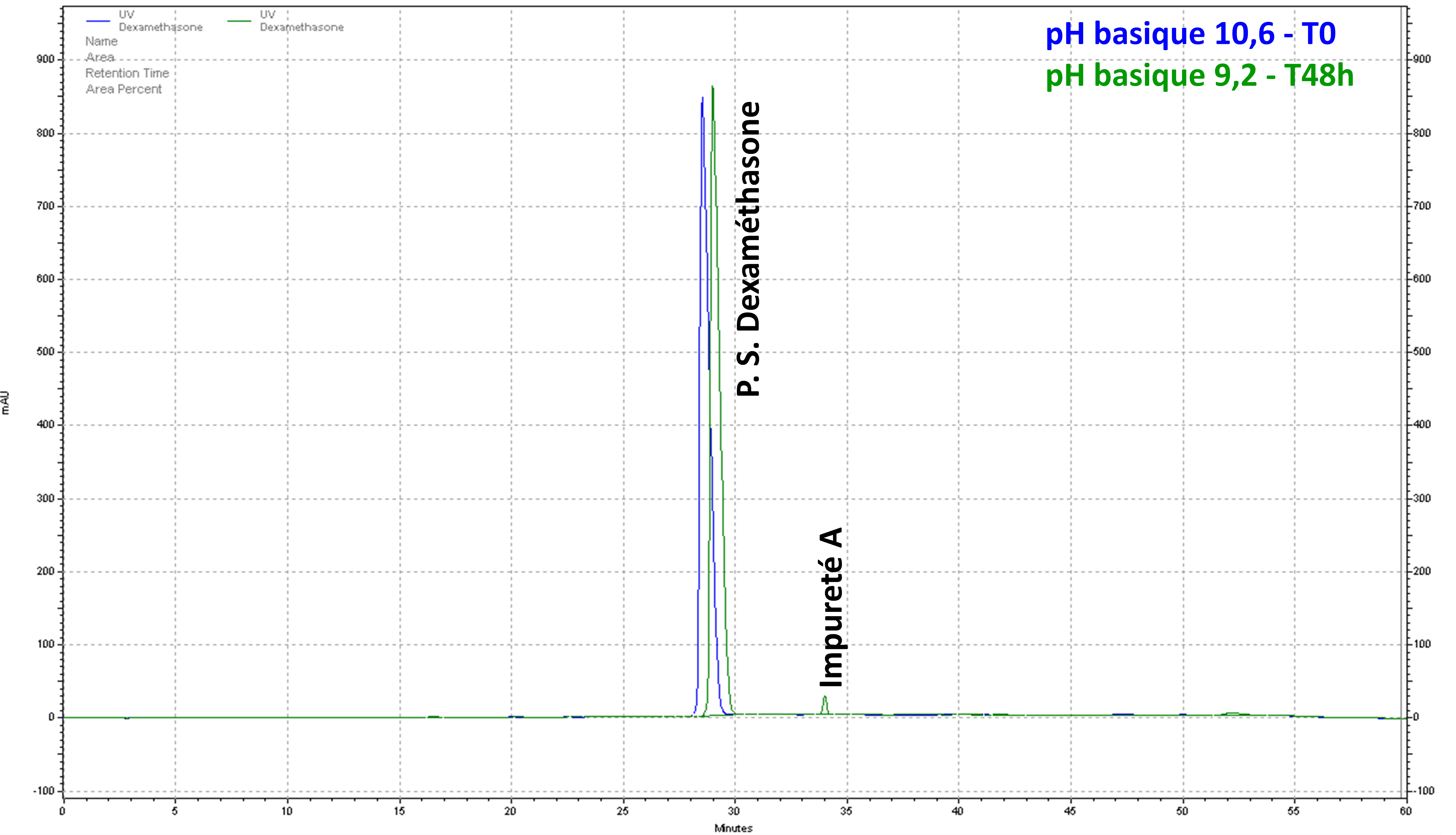
Chromatograms of dexamethasone sodium phosphate (1 mg/mL) at basic pH at T0 (blue) and T48h (green)
|




