|
Introduction
Nowadays, more and more dental implants are placed and during this operation, antiseptics are used, for example the chlorhexidine. Studies are done to improve the effectiveness of this antiseptic. To do that, researchers try to combine it with a metal because some metals have anti-bacterial properties. Many complexation methods are made and the first goal of the laboratory is to obtain single crystals to get their structure but for the moment none has been formed. Perhaps this is because the crystallisation is long. As a consequence, as single crystals are not obtained, several analyses were made to see whether a complex was formed or not. Moreover, chlorhexidine is only partially soluble in solvents so it is a problem to use it.
Experimental conditions
Many analyses, such as IR and UV-visible, were realised to check if there were complexations.
Firstly, IR spectrophotometer was used in order to make an IR analysis.
Different compounds were synthesised from copper and chlorhexidine but the colour of the precipitate obtained varied depending on the various bases used. Maybe that is the reason why all the complex obtained were different.
Secondly, UV analysis was realised with a Perkin Elmer Lambda XLS+ spectrophotometer. In the beginning, the copper metal was analysed alone. Then, a range calibration was made by adding chlorhexidine which acted as the ligand. The solvent was acetonitrile.
Results
IR analysis:
With IR analysis, it was impossible to conclude that a complex was created due to the complexity of the ligand. Indeed, chlorhexidine is a molecule which contains a lot of chemical functions, so, on the IR spectra, there were a lot of absorption bands and when we superimposed these bands, it was not very easy to see the differences. (Figure 1)
With this analysis, it could not be assumed that a bond was created. Indeed, the Cu-N bond appeared between 400 and 600 cm-1 theoretically but we cannot be sure that it was the case in this experiment.
UV analysis:
During the addition of the ligand in all metal solutions, the absorption increased (Figure 2). Moreover, the colour of the solution became yellower and the ligand became more soluble in the solution. That is the reason why it could be assumed that a complex was formed.
After this analysis, a hypothesis was made, which was that the complex was formed in the 1:1 ratio (when the ligand was in excess; it was less soluble in the solution).
Conclusion
Chlorhexidine is a ligand which is not easy to use because it is partially soluble in the solvent. As a consequence, some particles stay in the solution. Moreover IR analysis did not give any information about the complexation. However thanks to the UV analysis, when chlorhexidine is with a metal, this ligand become more soluble and that a complex is formed between these two compounds. Today, the crystallisation is ongoing.
|
|
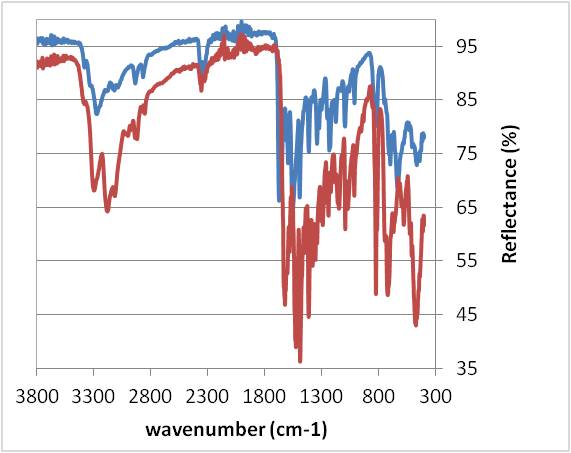
Overlapping of IR spectra: a: chlorhexidine alone (red curve)
b: synthesised compound (blue curve)
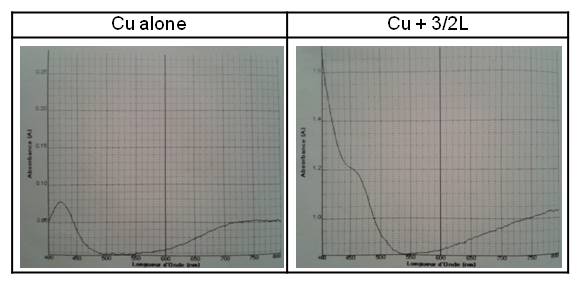
UV-visible spectra.
On the left: Cu alone. A=0.0775
On the right: Cu+3/2Ligand. A=1.2
|




