|
Introduction
Colchicine intoxication does not happen very often, but can be extremely dangerous when it occurs. Colchicine is used for the treatment and the prevention of gout flares and some inflammations, thanks to its anti-inflammatory effect. If ingested in high doses it can cause severe intoxications and/or death if not treated quickly, depending on the amount consumed. Thus, quantifying colchicine hastily is essential to find a course of treatment. The analyses were conducted on a liquid chromatography high resolution mass spectrometer (LC-HR-MS) Q-Exactive, by spiking whole blood with known concentrations of colchicine and the use of internal standards. The purpose of this study was to develop, validate and apply a method to quantify colchicine in whole blood.
Experimental conditions
Molecules were detected using a heated electrospray ionization source (HESI) in positive mode. Calibration curves were obtained by spiking human whole blood with known concentrations of colchicine between 0.30 and 20 ng/ml. To enhance analytical accuracy, an internal standard (Risperidone-D4) was used. Then solutions were extracted using a liquid-liquid extraction and afterward evaporated to dryness at 50°C, finally they were reconstituted with 200 µL of diluted solution and injected in the LC-MS system. Data was collected in Full Scan MS mode at m/z 400.1758 for colchicine and at m/z 414.5180 for the internal standard risperidone-D4. The data acquired was processed with the Xcalibur software.
Results
The assay was linear between 0.30 – 10 ng/ml, as shown in figure 1, with acceptable extraction recoveries. The retention time was 5.04 min for colchicine. This calibration curve will be used to quantify colchicine henceforth. The validation process of this method of quantification studied linearity, precision, extraction efficiency, process efficiency and matrix effect. The results of this validation are shown in Table 1 with two concentrations 3 and 10ng/ml of colchicine. The matrix effects were acceptable and process efficiency and extraction efficiency were satisfactory. The run time was 15 minutes.
Conclusion
This experimental study shows that the colchicine quantification in whole blood is possible by LC-HR-MS using the Full Scan mode.
|
|
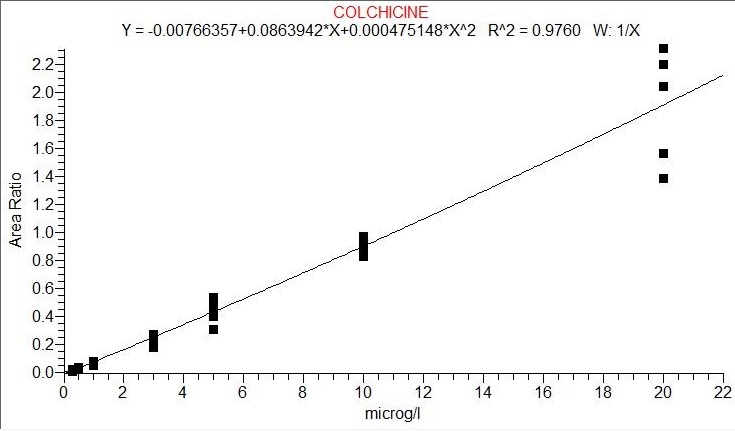
Figure 1 : Calibration curve of colchicine, from 0.3 to 20ng/ml
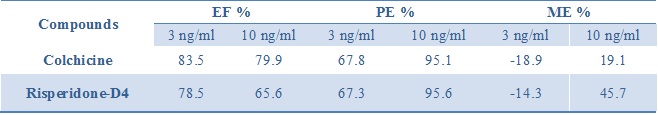
Table 1 : Matrix effects for two concentrations of colchicine, 3 and 10ng/mL Extraction efficiency (EF %), process efficiency (PE %) and matrix effect (ME %) (Data are mean of n = 2 analyses).
|




