|
Introduction
Column temperature is not usually considered as a key factor for LC separations. Separations are usually carried out at room temperature and mobile phase composition is one of the most important parameters. Moreover, in RP-HPLC, thermal instability of silica-based columns limits higher temperatures.
However, the elution of compounds is characterized by its equilibrium constant which depends on temperature. Thus, the aim of my experiments was to study the effects of temperature on retention and selectivity in RP-HPLC.
Experimental conditions
The chromatographic system was an Agilent Technologies 1260 Infinity Series, equipped with: a G4220A binary pump system, a G1316C thermostatted column compartment, a G4212B diode array detector set at 254nm, a G4226A autosampler with an injected volume adjusted to 1μL. The range of temperature in this study was from 20°C to 50°C, due to a maximum operating column temperature of 60°C. The Agilent Eclipse plus C18 bonded-phase that was used was 2.1x50mm, and had an average particle size of 1.80μm. The flow rate was fixed at 0.4mL/min and NaNO3 was used as the dead time marker. The solutes were benzene, nitrobenzene and βnaphtol. The retention factor k for a compound was calculated according to equation 1, where tr is the retention time and t0 the column dead time.
(Eq.1) k= (tr-t0)/t0
Results
The Van't Hoff equation provides a relationship between the equilibrium constant K and temperature T. By replacing K with the retention factor, according to Eq. 3, the influence of temperature on retention is given by Eq.4.
(Eq. 3) k= K/β
(Eq.4) Ln k= (-∆G°)/RT-Lnβ
(β is the phase ratio, ∆G° is the Gibbs free energy and R is the ideal gas constant)
The variations of Ln k with temperature are given in Fig.1 and 2.
Linear behavior was observed whatever the solvent used (MeOH or ACN). Considering the slopes of the Van’t Hoff curves, retention factor k decreased about one percent every degree depending on the solute and on the mobile phase. There was no improvement on selectivity with acetonitrile, and the pair which was difficult to separate at 20°C was not able to be resolved any better at 50°C. With methanol as the organic solvent, the temperature changed selectivity and the higher the temperature the higher the resolution.
Conclusion
Column temperature plays an important role in RP-HPLC: control of retention and control of selectivity. Moreover because temperature results in faster separations, analysis conditions can require less solvent.
|
|
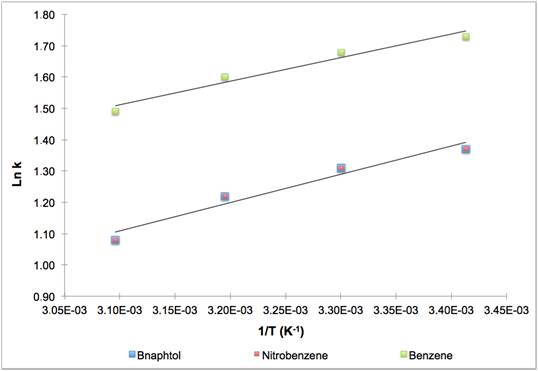
Variation of Ln k versus 1/T with ACN 40% / H2O 60 %
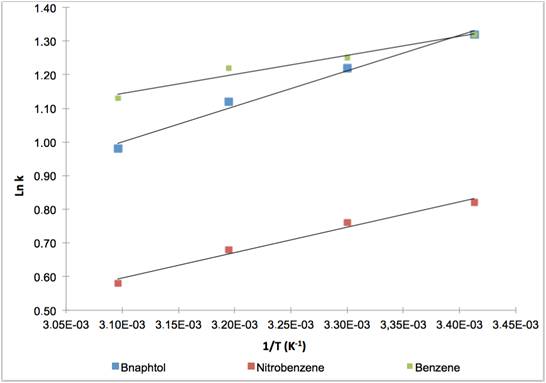
Variation of Ln k versus 1/T with MeOH 55% / H2O 45%
|




