|
Introduction
For some years, high energetic compounds have been introduced into our society by human activities. Used for civil and military applications, explosives prove to be environmental pollutants. Although these compounds, such as Nitropyrazoles, are frequently used, toxicity studies on human health have never been conducted until now. As part of REACH program, the toxicological laboratory of HCL was asked to study Nitropyrazoles' cytotoxicity.
Thus quantitative method for Nitropyrazoles was developed using LC/MS-MS. This article focuses on 3,5-Dinitropyrazole (3,5-DNP) and 3,4,5-Trinitropyrazole (TNP).
Experimental conditions
Experiments were carried out using an HPLC system Finnigan Surveyor and a Hypersil GOLD C18 column (100mmx2,1mmx3µm).
Chromatography was coupled with a mass spectrometer Finnigan TSQ Quantum Ultra made of an electrospray ionisation source used in negative mode and a triple quadrupole analyser. Some parameters were set:
- Injection volume: V=10µL
- Column temperature: T=30°C
- Flow rate: D=200µL/min.
Two steps composed this study. During the first development part, pure solutions of nitropyrazoles were prepared and directly injected into the mass spectrometer using two different mobile phases: MeOH/H2O + 0.1% acetic acid and ACN/H2O + 0.1% acetic acid. This part allowed characteristic transitions to be found in multiple reaction monitoring mode for each compound. After that, chromatography was coupled with the mass spectrometer and separation was optimized.
Results
With both mobile phases, same characteristic transitions were found for each molecule. 3,5-DNP had only one characteristic transition, m/z=157->65, whereas TNP had two: m/z=202->110 and m/z=202->64.
After testing for several compositions of both mobile phases, elution of 3,5-DNP and TNP was carried out using a binary gradient MeOH/H2O + 0.1% acetic acid.
It appears that these nitropyrazoles had a similar behaviour in chromatography (Fig. 1). Thus, 3,5-DNP could be used as an internal standard for TNP quantification. Reciprocally TNP could be the internal standard for 3,5-DNP quantification.
Conclusion
To sum up, the first step of this method development for 3,5-DNP and 3,4,5-TNP quantification was achieved. Nevertheless parameters, such as flow rate, gradient, collision energy, have to be optimized. Moreover, this method must be validated.
|
|
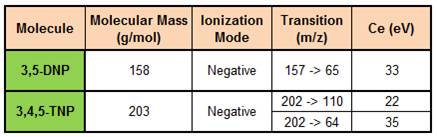
Detection conditions of 3,5-DNP and TNP.
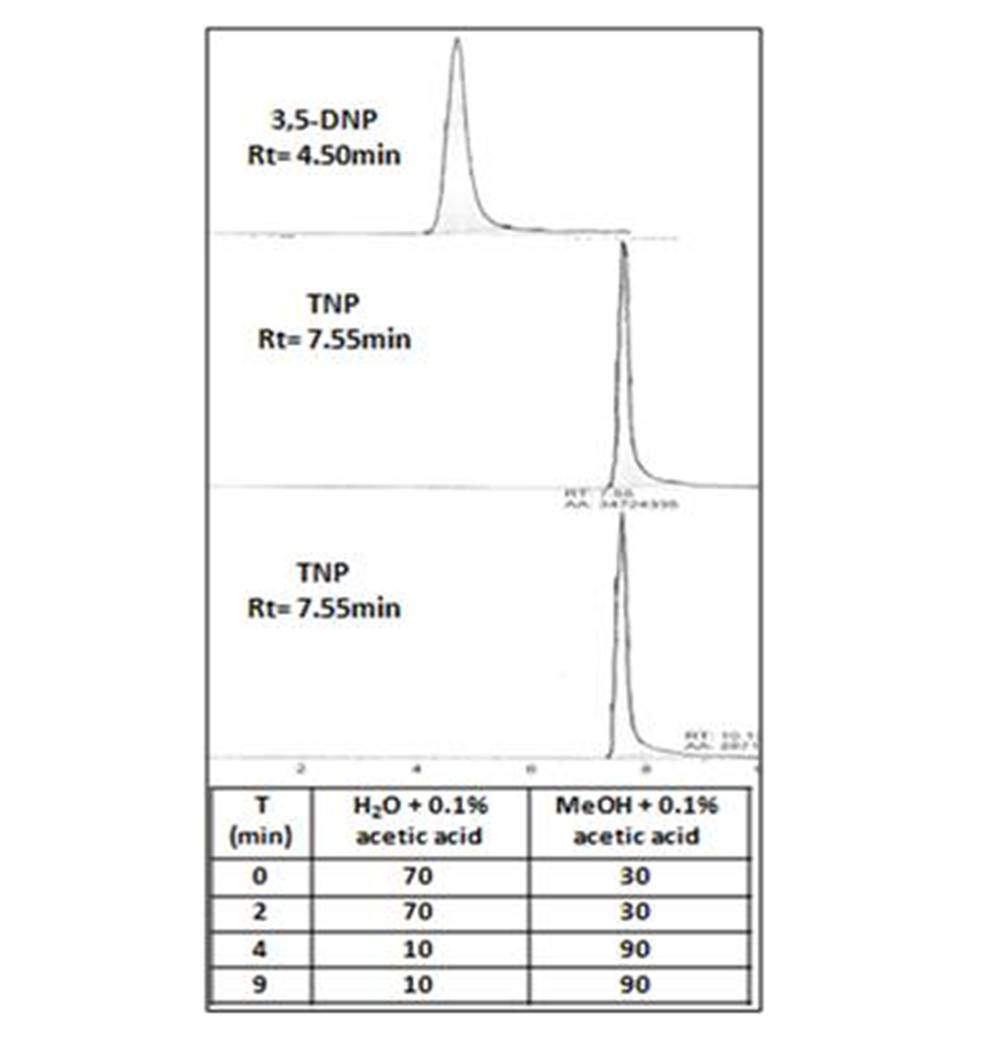
Chromatogram of 3,5-DNP and TNP separation.
|




