|
Introduction
The laboratory of industrial analysis of Solvay, situated at the Research and Innovation Center of Lyon (CRTL) in Saint-Fons enables to run and to set up online analyses on pilots and processes as well for external clients as for different departments of the group. The clients are looking for solutions in order to analyze what is really happening at different stages of a product fabrication or transformation. The point is to obtain as much information as possible so as to improve and control the process.
The purpose of this study is to calibrate a µGC in order to follow the concentration of diisopropyl ether into a process. The exponential diluter is used to generate the different calibration dilutions.
Experimental conditions
The exponential diluter is an analysis instrument that is used to vaporize a liquid composite onto a gaseous compound. Furthermore, it permits to dilute the composite exponentially and to do a whole calibration with only one injection.
The dilution law is C = Co.e-(D*t/V) with D the circulating flow, t the time, V the diluter volume and C0 the initial concentration.
The µGC is a device for separating and quantifying the injected gases.
First, to calibrate the µGC, a few microliters of diisopropyl ether were propelled onto the diluter which was then a closed system. The liquid was thus vaporized because of the saturated vapor pressure, and then diluted. The valves were opened and allowed the flow onto the diluter of argon which was the diluting gas. The diisopropyl ether was diluted exponentially owing to the argon flow. In fact the system was opened and the mixture came out of the reactor through an exit. The mixture was then sampled and analyzed by the µGC, settled first.
Results
First, we obtained the exponential curve (Fig.1) which shows the decrease in the compound concentration in function of the time. The initial time is when we opened the valves permitting the exponential dilution. The peak area decline exponentially as we expected. The R² term is upper than 0.999 so we could utilize those analyses and chromatograms to do the calibration. We chose 8 points on our range to do the calibration (Fig.2) and the correlation term, R², we obtained is upper than 0.999 as well. The results were then satisfying.
Conclusion
The exponential diluter is a simple way to do a calibration of a liquid for low concentration of light products and to vaporize a liquid. Furthermore, it is also a cheap analysis instrument that consumes just a few products and almost no energy.
A problem came along afterwards: the process was under pressure and the µGC we were using to run the analyses was not air-tight.
|
|
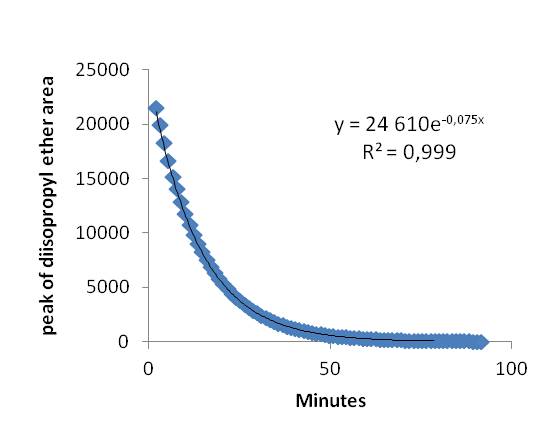
Figure 1 : Curve of diisopropyl ether diluted through the exponential diluter: the peak of the compound area measured by the µGC depending on time.
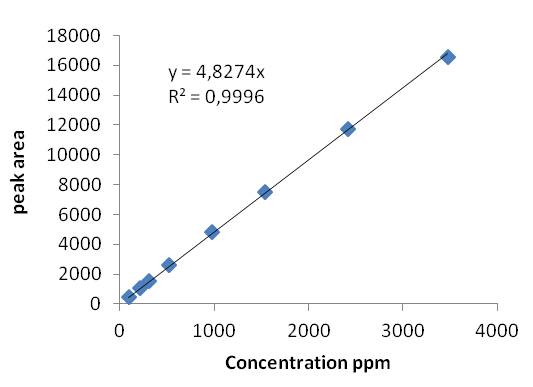
Figure 2 : Calibration of diisopropyl ether: the average area of the diisopropyl ether depending on the theoretical concentration (ppm) of the compound.
|




