|
Introduction
The concrete corrosion in sewer pipes is due to microorganisms in the biofilm which cover pipes and sulfur species in the network. Indeed, in wastewater there are sulfates which will be consumed by bacteria to form acids such as H2S or H2SO4 that will cause the concrete dissolution and the formation of alteration layers.
In order to monitor the deterioration, a method must be developed to quantify all sulfur species by ion chromatography.
In the samples which are small pieces of concrete covered with biofilm and alteration layers, there are different types of sulfur species: (i) water-soluble species, (ii) deterioration layers sulfates (acid-soluble) and (iii) elemental sulfur. The last one is neither soluble in water nor in acid. An extraction protocol must be developed.
Experimental conditions
All analyses are made with a Thermo ICS-1100 ion chromatography with a gradient:
Time KOH concentration
0-8 min 38 mM
8-18min 38-80mM
18-25 min 80 mM
In order to test the elemental sulfur extraction in the samples, an experiment is made with elemental sulfur powder, for 20 mg we add 100 mL of water, 1.5 mL of NaOH at 1 M and x =5, 10, 15 mL of a saturated solution of Na2SO4 and incubate overnight the whole at 60°C. The protocol is summarized in Fig. 1.
This process needs a high concentration in sulfites, which are unstable. They turn into sulfates. To obtain a calibration curve of sulfites, we have to quantify the sulfates and to calculate the mass balance to obtain the real quantity of sulfites.
Results
The quantity of thiosulfates created during the reaction between elemental sulfur and sulfites is negligible, that’s why we must minimize the amount of sulfites introduced to increase the detectability of thiosulfates.
The results are presented in the figure 2.
The more the volume of sulfites introduced decreases, the more the yield increases which can be explained by the fact that the detectability is better or that the elemental sulfur extraction is greater when the amount of sulfites is less important.
Conclusion
This process of extraction shows some difficulties: do a calibration with unstable compounds (sulfites), have a good detectability of thiosulfates although sulfites are in very large quantity.
However, with all the problems, a yield close to 50% was reached. By decreasing the amount of sulfites, the yield may increase considerably.
The result of this research in this protocol will be optimized to introduce the minimum of sulfites in order to have the best detectability and the best extraction yield.
|
|
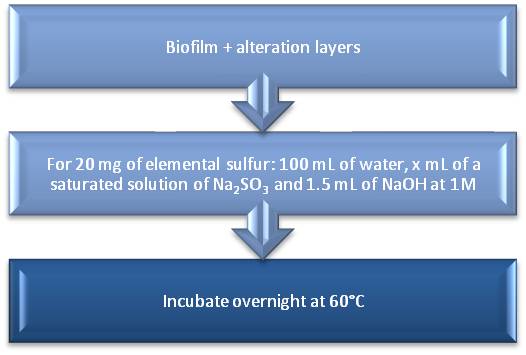
Protocol of elemental sulfur extraction
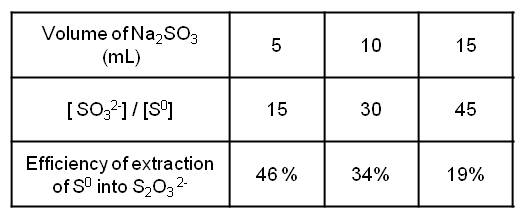
Results of variation of sulfite volume
|




