|
Introduction
This study in the research unit UMR 7245 of the CNRS and the National Museum of Natural History is the result of a collaboration with the CEA of Cadarache.
Some bacteria have the characteristic of having a surface protein layer, called the S layer, which protects against hostile conditions and in particular gives tolerance to high concentrations of metals and radionuclides. Our study focused on proteins of S layer of two bacterial strains, Lysinibacillus sp. 806 and 807, isolated from the Chernobyl soil. Thanks to a previous study, it is known that these proteins of 100 to 150 kDa are glycosylated.
Glycosylation is an essentially post-translational modification that adds glycans.
The main two types of glycosylations are :
- N-glycosylation : « N » means that the glycan is covalently attached to the nitrogen of the amide group of an asparagine.
- O-glycosylation : It is the bond established between either the N-acetyl galactosamine and the oxygen of the hydroxyl group of the serine (or the threonine), or between the galactose and the oxygen of the hydroxyl group of a hydroxylysine residue of the collagen.
The aim of my internship was to characterize these glycoproteins by a proteomic approach :
determine whether it is N-glycosylation or O-glycosylation
and then conduct a bottom up analysis by gel digestion and analysis by mass spectrometry.
Experimental conditions
In order to know the type of glycosylation, we used two methods of deglycosylation, one specific for N-glycosylation (enzyme reaction by N-glycosidase F) and one specific for O-glycosylation (chemical reaction of β- elimination).
Deglycosylated or non-deglycosylated proteins were analyzed by a 6% acrylamide polyacrylamide electrophoresis (SDS-PAGE) gel.
(The bands of interest were excised and subjected to the gel digestion by trypsin followed by the analysis by ESI-Q-TOF mass spectrometry (Maxis II ETD, Bruker).)
Results
Regarding N-glycosylation, by observing 806+ (treated with the kit) versus 806- (untreated), no difference was observed. Similarly for 807+ and 807-. It is deduced that the bacteria are not N-glycosylated (figure 1).
As regards O-glycosylation, for the 806+ and 806- proteins, no task was observed because these samples could not be placed at a pH between 6 and 8. In parallel, it is noted that the spots of the proteins 807+ compared to the 807- are less marked (figure 2). We can deduce that our strains are probably O-glycosylated, and we can not confirm it because we do not have control samples.
Conclusion
The bacterial strains 806 and 807 are not N-glycosylated. On the other hand, strain 807 is probably (because there is no control sample) O-glycosylated.
|
|
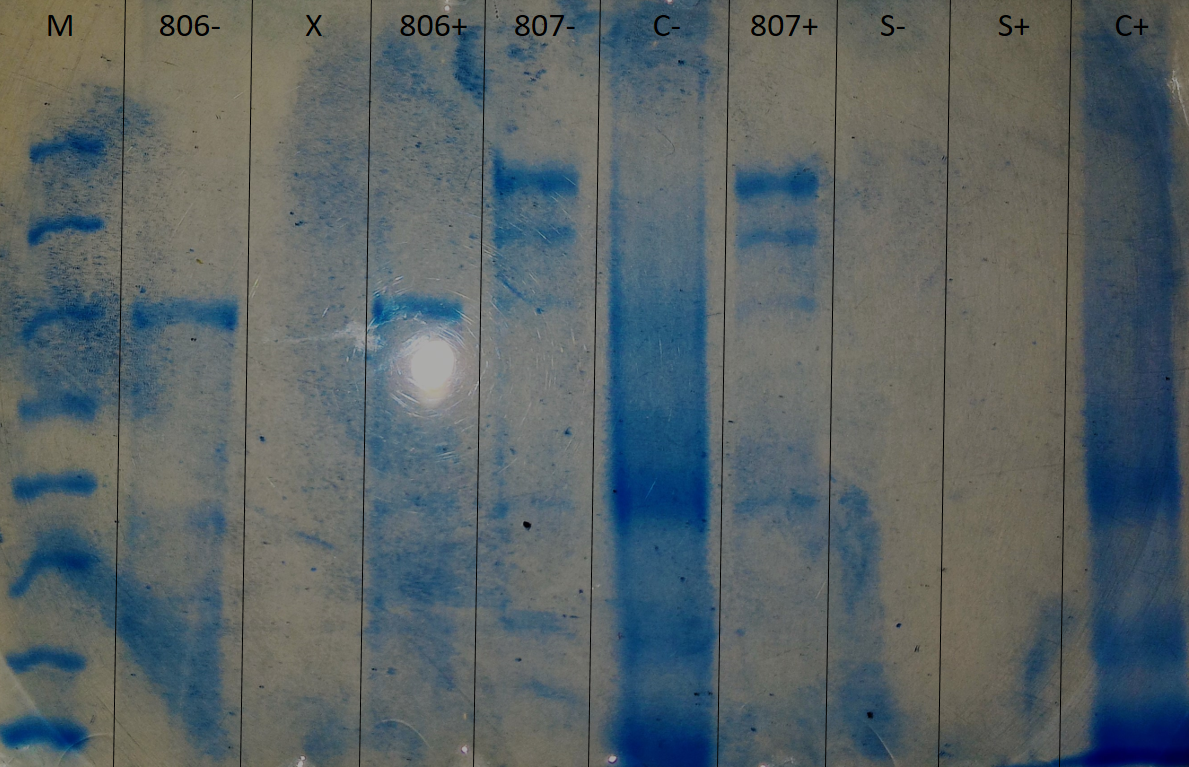
SDS-Page 6% polyacrylamide electrophoresis, executed to determine if the glycosylation is of the N-glycosylation type.
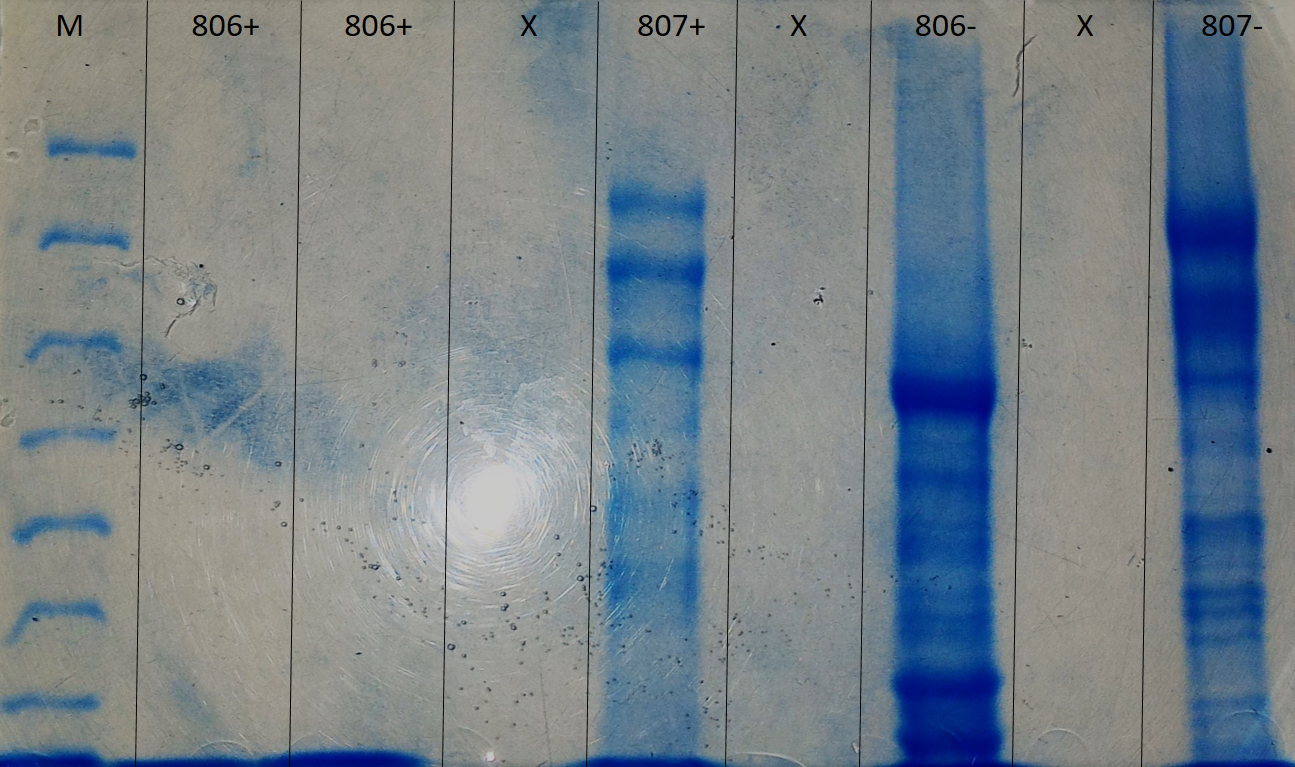
SDS-Page 6% polyacrylamide electrophoresis, executed to determine if the glycosylation is of the O-glycosylation type.
|




