|
Introduction
Opioid misuse and addiction is a serious public health problem affecting patients worldwide. The identification of a substance absorbed by an opiate consumer can turn out to be problematic, the reason for this being the lack of specific biomarkers for all the molecules comprised in this broad class of drugs.
Hence, there is a growing need for the certified identification of opioid analgesics and their derivates within Forensic Science and Toxicology laboratories.
This article will focus on the analytical validation of a fully-developed LC-MS/MS method destined to the quantitative analysis of 20 opioids in human plasma, whole blood and urine.
Experimental conditions
The separation of the 20 opioids was performed using a Dionex Ultimate 3000 high-performance liquid-chromatography system (Thermo Fisher Scientific, Germering, Germany) equipped with a X Select® HSS T3 (100 × 2.1 mm; 2.5 µm, WATERS) analytical column maintained at 60°C.
Online sample clean-up was performed using an Oasis HLB column (2.1 mm x 20 mm; 25 µm, WATERS). Each biological sample underwent a process of deproteinization using a sulfosalicylic acid solution spiked with the available deuterated internal standards. The injection volume was set to 50 µL.
The calibration curves were built by spiking drug-free biological matrices with known concentrations of opioid standards at 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20 µg/L for Buprenorphine, Norbuprenorphine, Buprenorphine-glucuronide, Norbuprenorphine-glucuronide, Fentanyl, Norfentanyl, Alfentanil, Sufentanil, Naloxone and Remifentanil and at 0.5, 1, 2.5, 5, 10, 50, 100, 200 µg/L for Methadone, EDDP, Tramadol, O-desmethyltramadol, N-desmethyltramadol, Oxycodone, Pethidine, Nordextropropoxyphene, Dextrometorphan and Tapentadol.
A API 4000 mass spectrometer (ABSciex, Toronto, Ontario, Canada) appointed with an electrospray (ESI) probe, allowed the completion of MS/MS analyses and was operated in positive mode. All analyses were performed in multiple reaction monitoring (MRM) mode and a full run was completed in 7.6 min.
Results
Selective and sensitive analytical methods for the quantitative evaluation of drugs and their metabolites are critical for the successful conduct of clinical toxicology studies and post-mortem investigations.
Along these lines, the verification and validation of this bioanalytical method has been entirely conducted in line with the specifications of the Food and Drug Administration (FDA). This included the determination of the specificity, accuracy, precision (repeatability, reproducibility, intermediate precision), contamination as well as stability under different storage conditions.
Therefore, the calibration curves obtained helped us to determine the target concentrations, especially of the internal quality controls, which were used for the validation.
Nevertheless, we encountered a sensitivity problem caused by a decalibration of one of the quadrupole mass analyzers in the mass spectrometer detection system. This led to an increase in the uncertainty of the measures which made it impossible for us to complete the validation.
Conclusion
This bioanalytical LC-MS/MS method destined to the quantitative assay of 20 opioids in human plasma, whole blood and urine was partially validated.
The results obtained are significantly interesting, especially when it comes to the newly added compounds related to this assay. This method allows a rapid and precise quantification of opioids in biological matrices, representing a very thorough assay that provides accurate and precise results.
|
|
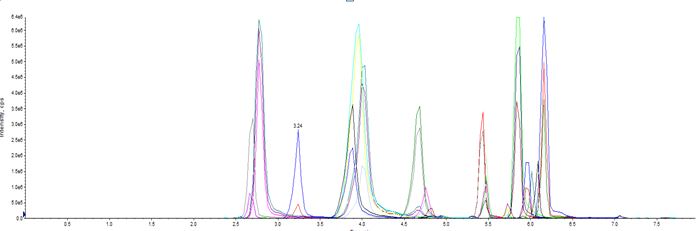
Chromatogram illustrating the separation of the 20 opioids in human plasma
|



