|
Introduction
Since the 2000s, the use of generic drugs is booming. Providing the same quality care as the original product and being subjected to the same regulations, generic drugs are generally less expansive from 30% for medications from pharmacies to 50% for hospital drugs. MAP France, a service provider, proposes the development of new drugs. A high performance liquid chromatographic method was validated.
Experimental conditions
In order to quantify the active ingredient, magnesium pidolate, an HPLC with UV detector was used. Operatory conditions are described below:
Instrument: Elite Lachrom Hitachi
Column: Aquasil C18 250mm*4.6mm*5µm
Flow rate: 1ml/min
Injection volume: 20µl
Solvent: pH 2.5 water acidified with phosphoric acid / methanol (2/98)
Detection: UV at 210nm
Retention Time: 7.30min
The method was validated according to the laboratory protocol. To perform this validation, it is necessary to define many parameters such as specificity, linearity, accuracy, repeatability and reproducibility. For all analyses, the API was diluted in water (0.75mg/ml). With the initial concentration (150mg/ml), the detector was saturated. Stock solution of API (3mg/ml) was prepared in water.
Firstly, a solution of all ingredients was analysed in the same proportion as the drinkable solution.
For the linearity, five standards from 80 to 120% of API concentration were prepared. Two linearities, one in water and one in matrix, were realised. All standards were conducted in triplicate. The matrix was composed of:methylparaben, propylparaben and saccharin (see figure 1).
Parabens were prepared in warm water to allow better dissolution.
The repeatability of the method was examined by injecting the solution consisting of magnesium pidolate (0.75mg/ml) into the HPLC system for three consecutive days. The reproductibility was tested with 10 injections on the same day.
Results
Firstly, the specificity was evaluated. A drinkable solution was diluted in demineralised water and injected.
The detection of the active ingredient was easily performed (Fig.2). The typical excipients included in the drug formulation did not interfere with the specificity of the method.
Secondly, the linearities were determined to control the matrix effect. Statistical analysis by the student’s test showed no significant difference between the two linearities. The accuracy was calculated from previous assays.
Finally, the reproducibility and repeatability coefficient of variation (CV) were less than 2% which is the limit set by pharmaceutical standards.
Conclusion
The analytical method was successfully validated. Indeed, all the validation criteria were approved for magnesium pidolate. The method was linear with and without any matrix. The routine analyses will be realized in water. The HPLC method allowed analyses to be repeatable, reproducible and accurate for the target concentration. A stability study could be carried out in order to verify API stability and the degradation of the active ingredient.
|
|
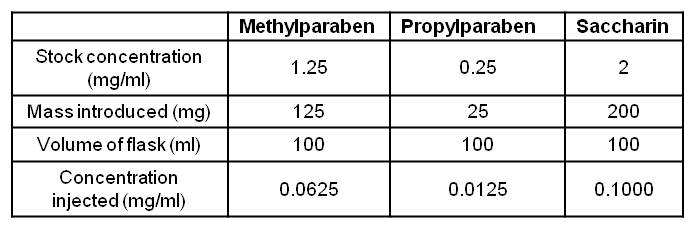
Preparation of the matrix
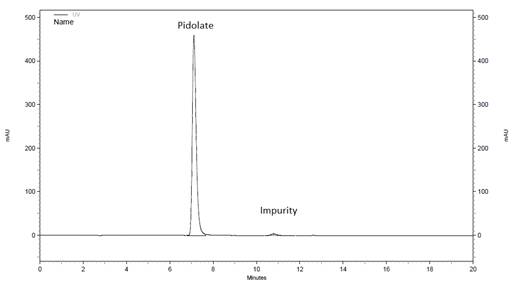
HPLC chromatogram of magnesium pidolate (0.75mg/ml) in water
|




