|
Introduction
Ionic chromatography is used in hydrology laboratories to find and quantify anions and cations in water. Chlorate, chlorite and bromate ions are very difficult to quantify because they are not sensitive enough. In order to improve the method, we need to find a good compromise between temperature and the flow affected to the ionic chromatography column. Increasing temperature allows a better resolution to separate those difficult ions. Increasing the flow provides a gain as well in time analysis.
Experimental conditions
We analyzed a sample with 1mg/L of chlorite and chlorate and 0.5mg/L of bromate which are the concentrations of the top of the range that we used. This sample is analyzed with an ionic Metrohm chromatography equipped with a Metrohm Metrosep A Sup 7 column and a Sodium hydrogenocarbonate and Sodium Carbonate mobile phase. First, we compared three temperatures 45/50/55°C of the column oven and we visually measured the optimal resolution for the chlorite, chlorate and bromate. Afterwards we tried two flows, 0.7ml/min and 0.8mL/min. We could not increase the flow more than 0.8mL/min because the column could not support the pressure for those kinds of flows. We measured the resolution and the retention time for the last compound which corresponded to the time of the analysis per sample.
Results
The separation was better at 45°C between chlorite, bromate and chloride and it was the same between bromide and chlorate. The resolution grew when the temperature decreased. Regarding the flow, there was no effect on the resolution, only on the time of analysis. Time of analysis with 0.7mL/min was 35 minutes and consumed 24.5mL of solvents per sample. About the flow at 0.8mL/min the duration was about 30 minutes and consumed 24mL of solvents
Conclusion
Varying temperature might affect the resolution between ions. With a temperature of 45°C we could separate and quantify chlorite ions, chlorate and bromate. Furthermore, we fixed a 0.8mL/min flow because it gives the best analysis time and consumed less solvents. Increasing the flow provides an economy of solvent and rapidity to the analysis, so we can launch more samples per run with the same volume of mobile phase. In these conditions, the method must be optimized with the new column.
|
|
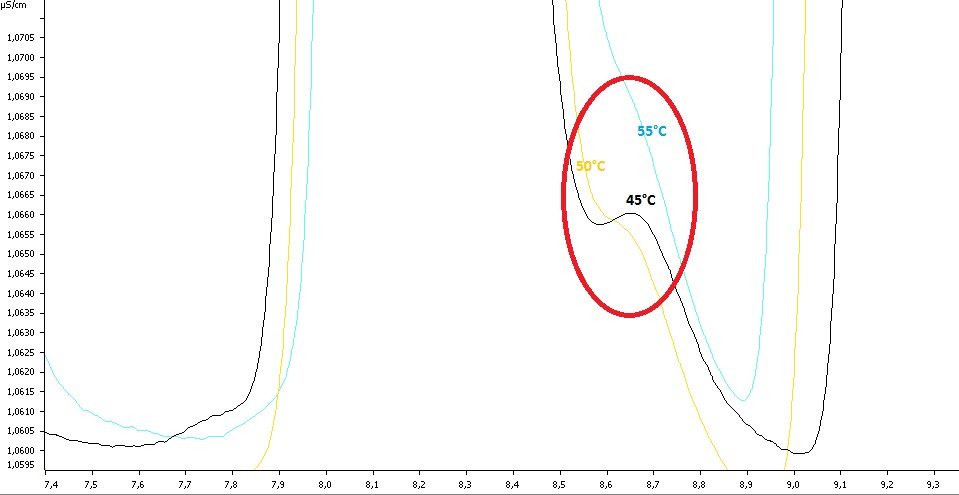
Chromatogram representing separation of chlorite, chlorate and chloride at three temperatures 45/50/55°C.
This is a superposition of three chromatograms zoomed on chlorite, chloride and bromate.
The first ion eluted is the chlorite then it is the bromate which is circling in red and the last one is the chloride.
In black we have the chromatogram at 45°C, 50° in yellow and 55°C in light blue.
|



