|
Introduction
Breath tests allowed the non-invasive exploration of several digestive and hepatic functions. The carbon 13 is the main isotope used like tracer because of its non-radioactivity. The carbon 13 measurements in breath are performed by a sensitive and specific method: the isotope ratio mass spectrometry (IRMS).
The 13C-urea breath test is the most available, for the diagnosis of gastric Helicobacter pilory infection. This test has been recently taken in charge by the national health insurance. Different tests are also available to study gastric emptying and intestinal transit time.
Experimental conditions
The principle is simple. For the Helicobacter pylori test, we administrated orally urea to the patient. This urea is marked by a carbon 13 atom which is stable. We used urea for this test because of the intense urease activity of Helicobacter pylori. After the ingestion of the urea, this one is hydrolysed to ammonia and carbon dioxide, which is diffused into the blood and is excreted by the lungs. The patient blows up a tube and the isotopically labelled 13CO2 can be detected in the breath by IRMS.
IRMS is certainly a very sensitive method and when it is coupled to a gas chromatographic column, the analysis is highly precise. IRMS measurements yield the information of isotopic abundance of the analysed gas relative to the measured isotope ratio of a reference gas.
This instrument is different from conventional mass spectrometers, indeed it doesn’t scan a mass range for characteristic fragment ions in order to provide structural information on the sample.
Three main sections compose the IRMS: ion source, mass analyser, ion collection. The ions are collected by three Faraday detectors which correspond to three ions traces for the different isotopomers:
m/z=44 12C16O2
m/z=45 13C16O2 12C16O17O
m/z=46 12C16O18O 12C17O2 13C16O17O
The ion currents are finally transferred to a computer which integrates the peak area for each isotopomer and calculates the corresponding ratios.
Validation of the method
In the aim of the validation, we needed to test the repeatability and the intermediate precision. We cannot do any other experiments because the sample is the air. So there is no dilution, interference, reactives …
A comparison with the Edouard Herriot Hospital (HEH) old method has been done. For this, they have sent us the samples of ten patients, which were already analysed by HEH. We have analysed a second time these samples and compared our results with HEH’s.
Results
The repeatability and intermediate precision results (Figure 1) are admissible because we found a standard deviation of 0.38 compared to 2.89% in scientific publication and 2.73% compared to 21.6%.
The comparison with an old method was good (y=0.9788x-1,7052 ; R2=0.9698) and when we performed the inter laboratory comparison, all the results of our laboratory were in agreement with the other laboratory.
Conclusion
To conclude, the IRMS is a highly accurate and specific breath test analysis, and it is excellent to use it for routine analyses. Furthermore, it requires not much maintenance. The only disadvantage is its cost.
|
|
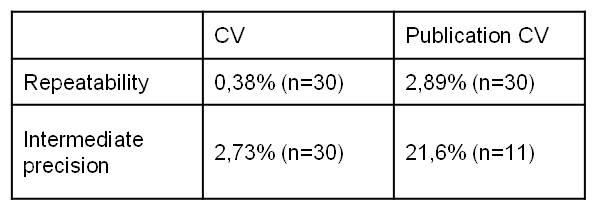
Precision results
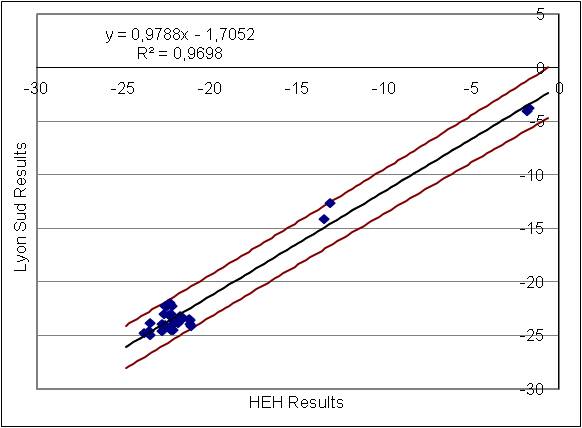
Dispersion Chart
|




