|
Introduction
From an ethical and regulatory point of view, the pharmaceutical industry needs to produce and market high-quality medicines. This obligation requires the establishment of a quality assurance system at all levels of the company. To reach this quality, the need for a validation and qualification arises in order to reduce the risk of non-compliance. It is essentially governed by good manufacturing practices. Validations concern processes, analytical methods and cleaning methods, while qualifications concern equipment and machineries. A new dishwasher installed in a laboratory must therefore be qualified and validated before it can be used. The protocol below was written and analyses were carried out to reach this qualification and validation.
Experimental conditions
Laboratory dishes were contaminated with 5 mL methylparaben (PHBM) solution at 10 mg/mL. One component of each type of laboratory dishes was contaminated but not cleaned; it served as a control for contamination.
All components were analyzed by UV-spectrometry (200-400 nm) and compared to a reference solution of methylparaben at 1 ppm.
The pH of purified water was measured in an element of washed dishes and compared to the pH of purified water in the same element but rinsed several times with water.
Results
To have an effective washing, the UV-spectroscopy needed to show a concentration below 1ppm of methylparaben and a second pH measurement 0,5 below or above the first one.
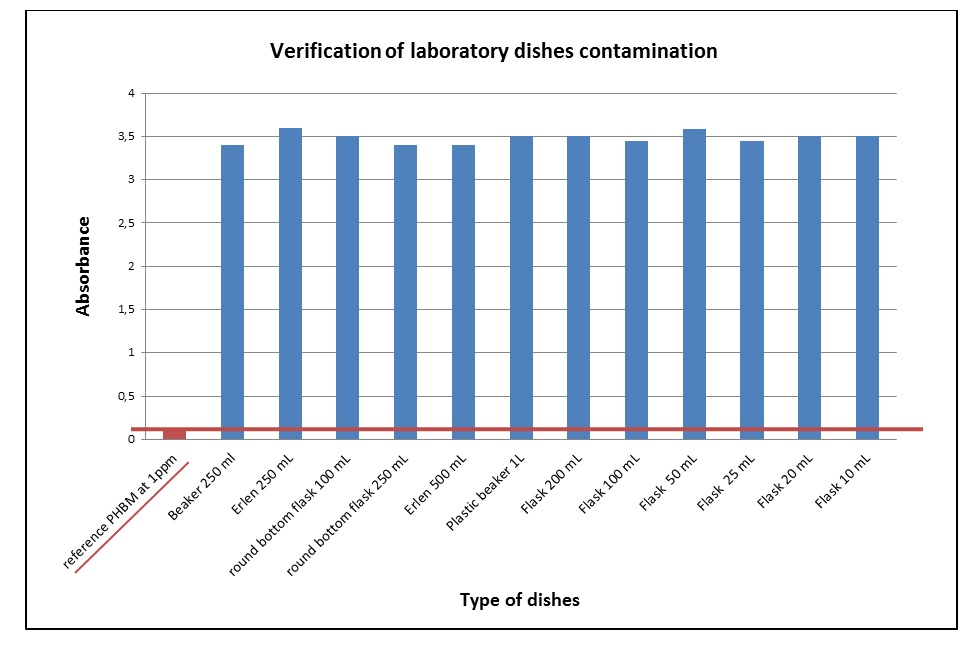
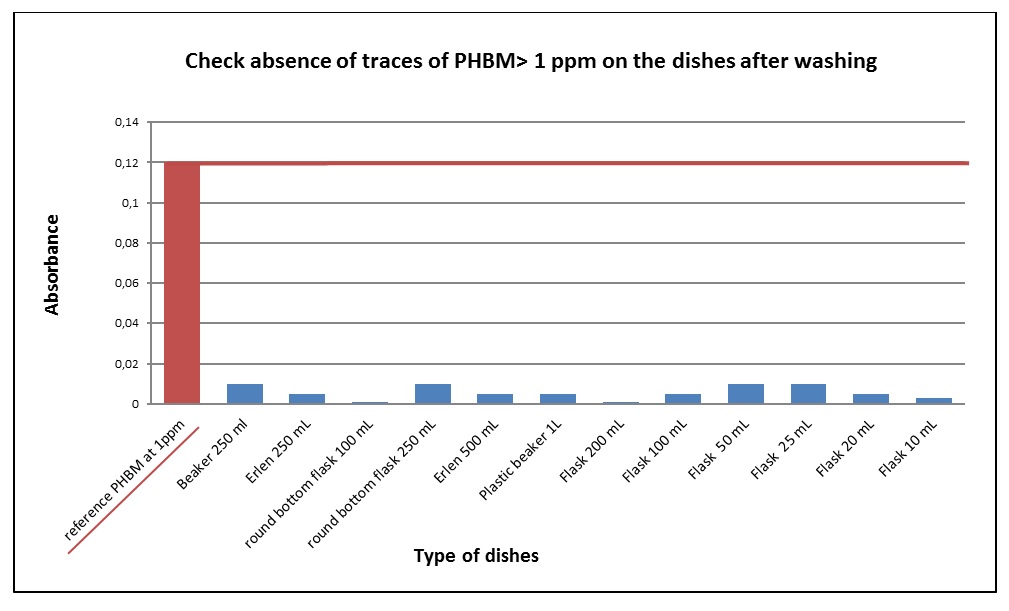
Results of UV-spectrometry analysis show that dishes were contaminated with methylparaben at a concentration greater than 1 ppm (Figure 1). Furthermore, after being washed, all laboratory dishes contained less than 1 ppm of methylparaben (Figure 2). This shows that the washing was effective.
The gap between two pH measurements was 0,3.
Conclusion
Among the three validation cycles, all components of laboratory dishes contained less than 1 ppm of methylparaben. The first acceptability criterion was accepted. The second criterion, which is the pH, was also accepted (ΔpH = ± 0.5) so the cleaning method was validated .The performance qualification was validated by the cleaning method.
|