|
Introduction
Microcalorimetry is a science that measures heat exchanges associated with chemical reactions or physical transformations, as a function of temperature and time, on very small sample volumes. The TAM method used is non-specific, thus, the measured heat fluxes contain all the contributions of the different reactions that have occurred in the material.
Its principle is relatively simple: the exothermic reaction generates a heat flux. The apparatus compensates this flow by cooling the sample by Pelletier effect, in order to bring it back to the working temperature. The current required for the Pelletier effect is directly proportional to the heat flux produced. The shape of the curves obtained will thus make it possible to establish reaction advance profiles, but also to understand how the rate of reaction varies as a function of the progress of the latter.
Experimental conditions
To measure a heat variation, the sample must be compared to a reference. Here, the reference used is a sand sample of 11.21 g of calorific capacity equal to 8.407 J.K-1.g-1. In order to perform the test, the sample and reference must imperatively have the same calorific capacity. It was therefore necessary to determine the quantities of sample (powder + solution) to be used to satisfy this condition. Once placed in the calorimeter at 20°C, the tests are left for several days in order to observe the progress of the reaction. A selection of 4 samples of alkaly-actived material was carried out with Na2O/ Al2O3 ratios ranging from 0.6 to 1.2.
Results
The opposite graph shows the heat flow evolution as a function of time for these 4 samples. There are two peaks common to all curves. The first peak is called "wetting peak". It is fine and intense, and represents the first high heat release when the aluminosilicate powder comes into contact with the activating solution. The second peak, wider and less intense, represents the "peak of reaction". It conveys the reaction rate of the material over the first few hours. This is the most significant one; it shows that the quantity of added soda has a direct effect on its intensity. Indeed, the more soda there is, the more species there are in solution, and therefore, there is a greater release of heat.
Conclusion
The quantity of NaOH makes it possible to intensify the peak of the main reaction involved in these products formation. But the intensity of this peak is all the more so important as the speed of reaction is rapid. As a result, sodium hydroxide makes it possible to accelerate the speed of this reaction, and hence the hardening speed of the material.
|
|
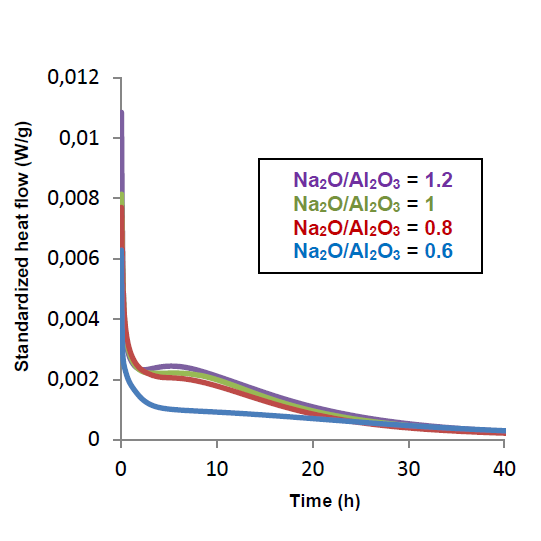
Curves of the heat flows obtained as a function of time, during the analysis of four samples of alkaly-actived material by microcalorimetry.
|


