|
Introduction
In this ENS research lab, many research projects are developed among which one that aims at synthesizing organo-metallic catalysts. A new family of helicenic molecules was chosen to play the role of ligand for the creation of the catalysts and more specifically the helicene called BisFHOMe (presented in table 1). We explored the coordination chemistry of this molecule using several different metal salts. NMR experiment were carried out to monitor the complexation. Data treatment then permitted to evaluate the association constant.
Experimental conditions
To perform these reactions, we first elaborated a procedure for the metal-ligand titration in ACN-d3. At the end of the titration, an excess of metal has been added in order to displace the equilibrium. This NMR titration is made on a Bruker 500 MHz NMR spectrometer by recording a 1H spectrum after each 10µL addition of the metal solution.
Results
For several metals and the helicene (table 1) we observe a variation of the chemical shifts that shows a coordination reaction that is characteristic of an equilibrium in fast exchange at the NMR time scale. The results are reported in the table 1.
The evolution of the the chemical shift for a given proton as a function of the concentration of metal is reported for two different silver salts (AgBF4 and AgOTf) in figure 1. Data treatment with the HypNMR software gives a extrapolated value for the association constant. The curve obtained from the titration with AgBF4 was well fitted and an association constant of 104 was computed.
For some metals, the expected coordination reaction competes with a protonation reaction, this is the case for CoCl2.6H2O and Pd (ACN)4(BF4)2. The protonation process is characterized by the obtention of the known NMR spectrum of the protonated helicene at the end of the titration.
Conclusion
The objective of this work was to monitor the coordination reactions of helicenic molecules. This was achieved with silver salts where high association constant was measured.
We have shown that for other metals the same experiment resulted in a protonation reaction instead of a coordination reaction. The reason for this could be that, due to their physico-chemical characteristics, those metals are not adapted for this type of ligand.
At the end of this work, after having screened several conditions, we have obtained a silver complex. The objective is now to find reactions in which this complex can be used as catalyst. This study also indicates that the coordination reactions in this ligand might not be as easy as expected due to a competition with a protonation reaction. Nonetheless the NMR titration method seems to be adapted to monitor the coordination process in this ligand.
|
|
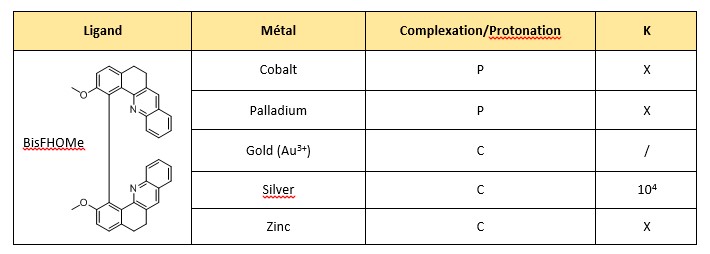
Results complexing tests with the helicene. P: protonation. C: coordiantion. /: no titration. X:no values
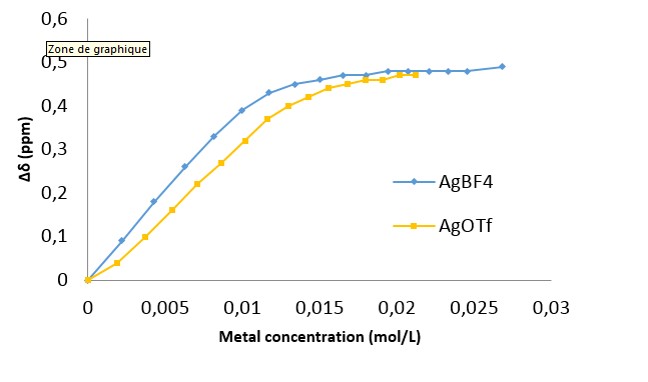
NRM titration for two kind of metal salt.
|




