|
Introduction
Breathing compressed air is a common practice for many professionals (divers, firefighters …). Air bottles are made of Oxygen, nitrogen, helium but may contain toxic gas such as carbon monoxide and carbon dioxide. To limit chemical toxicity risks associated with compressed air usage, laboratories need sensitive analysers (until 1ppm).
The analysis of trace levels of CO and CO2 can be problematic by gas chromatograph especially at low levels, that is why a sensitive equipment was developed. It consists of a Gas Chromatograph by flame ionization detection, preceded by a hydrogenating reactor, which converts CO2 and CO into methane CH4. The method is developed at the Navy laboratory to the analysis of CO2 and CO contained in compressed air.
Experimental conditions
For this separation, a Gas Chromatograph Clarus® 480 (from PerkinElmer®) with a column "Micropacked » Shincarb ST of length L=2m, internal diameter id=0.53mm were used. The vector carrier used was argon. The temperature ramps in this study were initially of 45 °C for 3 minutes high up to 110 ° C with a pressure of 7 psi and with an injected volume adjusted to 1mL.
After the separation on the analytical column, sample compounds passed over the hot nickel catalyst (temperature of 380° C) in the presence of hydrogen, where the carbon monoxide and carbon dioxide were converted to methane. The principle of the methanizer is based on the Sabatier reaction:
CO2 + 2H2 ↔ CH4 + O2
2CO + 4H2 ↔ 2CH4 + O2
">After the separation on the analytical column, sample compounds passed over the hot nickel catalyst (temperature of 380° C) in the presence of hydrogen, where the carbon monoxide and carbon dioxide were converted to methane. The principle of the methanizer is based on the Sabatier reaction:
CO2 + 2H2 ↔ CH4 + O2
2CO + 4H2 ↔ 2CH4 + O2
">For this separation, a Gas Chromatograph Clarus® 480 (from PerkinElmer®) with a column "Micropacked » Shincarb ST of length L=2m, internal diameter id=0.53mm were used. The vector carrier used was argon. The temperature ramps in this study were initially of 45 °C for 3 minutes high up to 110 ° C with a pressure of 7 psi and with an injected volume adjusted to 1mL.
After the separation on the analytical column, sample compounds passed over the hot nickel catalyst (temperature of 380° C) in the presence of hydrogen, where the carbon monoxide and carbon dioxide were converted to methane. The principle of the methanizer is based on the Sabatier reaction:
CO2 + 2H2 ↔ CH4 + O2
2CO + 4H2 ↔ 2CH4 + O2
Results
After the methanizer, CH4 could be detected by a flame ionization detector. The flame of the burner on the FID was fed with a mixture of dihydrogen and air, respectively with a flow rate of 45 and 450 mL / min. When these were burned, hydrocarbons produce ions and these ions were detected using a metal collector. The current across this collector was proportional to the concentration of hydrocarbons in the sample gas.
Conclusion
To conclude, this method allows the identification trace levels of carbon monoxide and carbon dioxide by conversion of CO and CO2 to Methane without changing retention times.
The catalytic reduction of CO and CO2 to methane was complete, accurate and repeatable, but this device requires temperature control, catalyst selection and sufficient hydrogen flow.
|
|
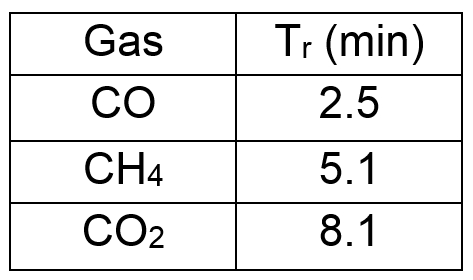
Retention time for 3 gas in this experimental condition
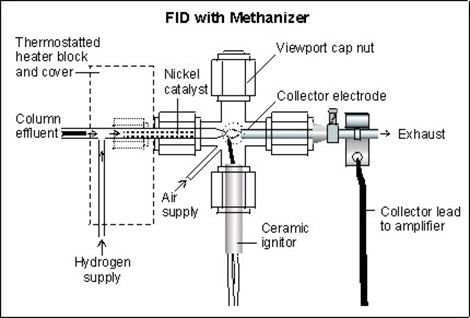
Diagram FID with the methanizer system used
|



