|
Introduction
Beyond routine analyses, the analyst may have to identify unusual and unknown samples. No general procedure has been developed so far to solve such a request because of sample diversity and complexity. An analysis needs to be thought considering the product nature and the laboratory equipment. In this way, we first of all have to ask if the sample identity is suspected or not. When it is, confirmation analyses can be carried out.
In this article, we will be dealing with a solid sample brought by the demining diver team of the French Navy to the Laboratoire d'Analyses de Surveillance et d'Expertise de la Marine (LASEM) in Cherbourg. It was collected from a shipwreck dating back to the 19th century. We made the assumption that the solid is cement considering its greyish appearance and its hardened texture. The aim of this study is to present an established approach within the laboratory to confirm or not the hypothesis.
Experimental conditions
A cement is typically composed of silica (SiO2), alumina (Al2O3), ferric oxide (Fe2O3), magnesium oxide (MgO) and lime (CaO). To achieve our goal, qualitative analyses were led to confirm the presence of these elements.
The first step was to convert the solid sample in a compatible form with the analytical equipment. For this purpose, the sample was crushed using a non-metallic hammer, then a mechanical grinder with agate bowls and marbles, and a porcelain mortar. When the sample was in a powder state, we could proceed to its solubilization by acid attack and heating, with a mix of hydrochloric acid (HCl) and nitric acid (HNO3).
The second step, after filtration of the attacked residues and rinsing with water, consisted in analyzing the filtrate using ICP-AES. The CID detector allowed a simultaneous acquisition of the characteristic lines giving a map of the elements in solution.
Results
Thanks to the ICP-AES analysis, we mainly detected the presence of zinc, magnesium and calcium in solution. We did not thus conclude on the sample identity because of the lack of detection of major cement component which is silicium, and low quantity of aluminum and iron.
The reason of the non detection should be an open question and require further analyses to know if it was linked to the absence of these components, or to an inadequate sample treatment. Indeed, we preferred, for practical and security reasons, to test the solubilization with HCl and HNO3 instead of using hydrofluoric acid (HF), the only one acid being able to attack silica. The solubilization with HF would necessarily lead to the silicium detection if the sample contains silica. Besides, solubilizating Al2O3 and Fe2O3 by attack acid may not lead to the elementary forms Al and Fe, normally detected using ICP-AES. However, this technique is usually used by our laboratory to analyze ground and sediments samples.
Conclusion
To conclude, the suspicion of the sample identity helps the elaboration of an analytical approach. Steps are easier to set up when we know what we are looking for. However, we have to consider all the necessary elements for the analyses. Sometimes, we have to admit that we took a wrong direction and must reconsider the situation.
|
|
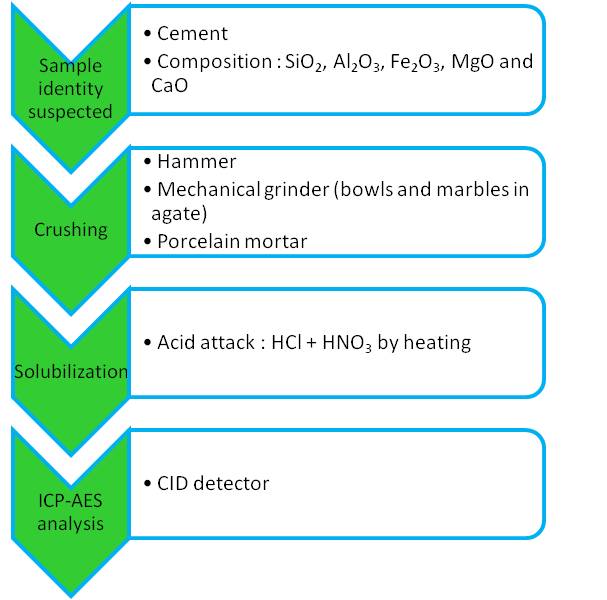
Analytical approach for identification of unknown sample declined in four steps from preparation of the sample to its analysis.
|



