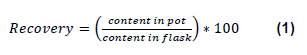|
Introduction
CTC laboratories are accredited to undertake analyses on leather, textiles, water and mud. One of the experiments they make consists in evaluating the content of metals which are extractable from a material due to a prolonged contact with sweat, simulating the effect produced if the product was worn by a customer. Up to now, the protocol for leathers and textiles were the same, following the standard ISO 17075-1. In 2015, the ISO 16711-2 was published regarding the textiles analysis. In consequence, a new protocol must be developed to fit the standard. However, the norm requires the use of flasks to carry out the extraction whereas the lab uses plastic tubs (pots) and wants to keep this container, for economical and practical reasons. It is therefore necessary to see if this change of container has an impact on the results. If not, it would not be illogical to keep the tubs at the expense of the norm.
Experimental conditions
First, five different textiles were selected for the experiment to have representative results. It was important to have the analytes in small pieces to increase the interface and so the extraction. 1±0.01g of each cut textile was put in both flasks and tubs. Then, 50ml of synthetic sweat were added in order to cover the textile with liquid. The extraction was performed using a hot water bath. The temperature, 37°C, was the same as the one of the human body and the solutions were stirred at level 3. At the end of the extraction, the solutions were filtered on filter paper and then on 0.45μm nylon membrane pores. The quantification of extracted metals was achieved using an ICAP-Q ICP-MS from Thermo Fischer, equipped with a quadrupole to select the ions of interest. Calibration standards covered concentrations from 0.01 μg/L to 1000μg/L and were prepared using a multi-element solution.
Results
The results reveal a difference between the extractions carried out in pots compared with the ones done in flasks. As it is summed up in Figure 1, metal concentrations are mainly lower if tubs are used. This result is the same for every textile of different composition. Furthermore, the figure shows that the problem of lower concentration in pots is not linked to a metal in particular.
Indeed, under-dosed elements in a textile are different depending on the sample. For example, iron recovery, which is calculated using the formula (1), is lower than 85% for textile 1(79%) and 3(81%), but reaches 95% for textile 2. Not all elements are presented here but the tendency is the same for.
Conclusion
It has been demonstrated that the container has an impact on metal concentrations analyzed by ICP-MS, since lower contents are obtained in pots. The container may interact with the extraction solution and may cause losses or, in some cases, pollutions. Presently, to fit the ISO 16711-2, the lab can not use these containers to perform the extraction since they cause systematic errors. The increase of the stirring velocity for tubs could be a solution to extract more metals, and additional assays must be conducted.
|
|
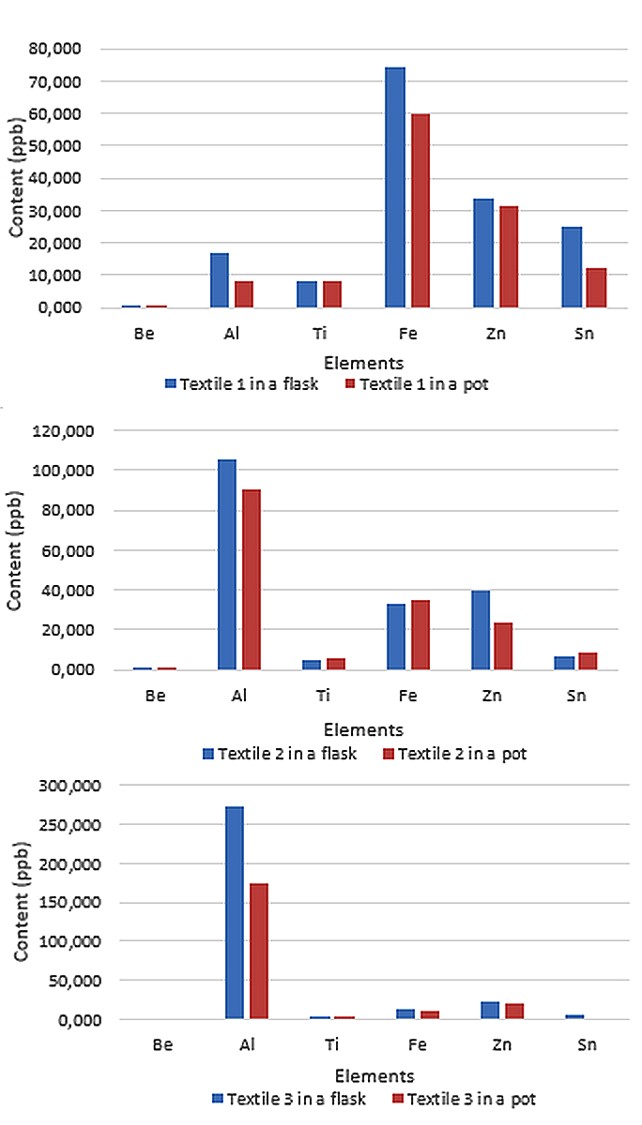
Figure 1:Results obtained after the extraction on three different textiles, named 1, 2 and 3, in both pots and flasks.
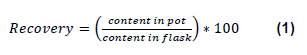
Figure 2: Formula
|