|
Introduction
The popularity of this technique has grown and gained worldwide acceptance for the analyses of alcohols in blood and residual solvents in pharmaceutical products. Headspace is a technique suitable for the quantitative or qualitative analysis of volatile or relatively volatile components in complex matrices.
HS analysis is based on the equilibrium between the liquid and the vapor phase where the compounds are. The total vapor is extracted and injected in the column of GC automatically, thanks to this appliance.
The goal is to concentrate the compounds by using the parameters of HS trap. Moreover, the sensibility of the response could be better when the matrix’s nature is changed with the addition of salts.
Experimental conditions
To see the influence of salts, all parameters of HS trap are optimized. The matrix was heated at 80°C. The experimentations were carried out, using a 1.4µm, 0.25mm x 60m Elite-624 column and a mass spectrometer with electron ionization as source, and simple quadruple as analyzer. The chosen compounds have the opposed physicochemical properties: polar (acetone, furfural, 2-butanol and MEK) and apolar (Toluene, éthylbenzène, m-xylene, o-xylene, chloroform). The selected salts were: NaCl, Na2CO3, KNaCO3, K2CO3, Na2SO4, and K2SO4. Then four grams of salts put in ten milliliters of water samples.
Results
The salts have a real impact on the area, so on the concentration of our compounds in the gas phase. For example, the MEK’s concentration is multiplied by 10 and the furfural’s concentration by 30, with the sodium carbonate in comparison to the solution without salts. Whereas the potassium sulfate has low incidence, because its solubility is only to 214 g/L at 80°C (compared to the solubility of K2CO3 which is to 1 400 g/L).
The salts reduce the constant of equilibrium. It gives specific properties to the solution. The efficiency depends on the ionic strength of the solution provided by salts. This effect increases when the solubility of salts and valance ions rise. This phenomenon is very important for the detection of polar compound, because the limit of detection and quantification decreases.
In order to know the best salts, the experiment with the same salts’ molar concentration and the same valence ions would be accomplished. In theory, a good salt is one which has a great solubility in sample and the largest ion size and the high ionic valence.
Therefore among the selected salts, the best one would be the potassium carbonate with a solubility on 1 400 g/L.
Conclusion
The salts have a real influence on the thermodynamic equilibrium between the liquid and the gas phase, that is to say the concentration of compounds in gas increases. So the method of organic pollutant analysis is optimized by the addition of salts. A good salt would be one which has a great solubility and gives ions with a high valence and a large size.
|
|
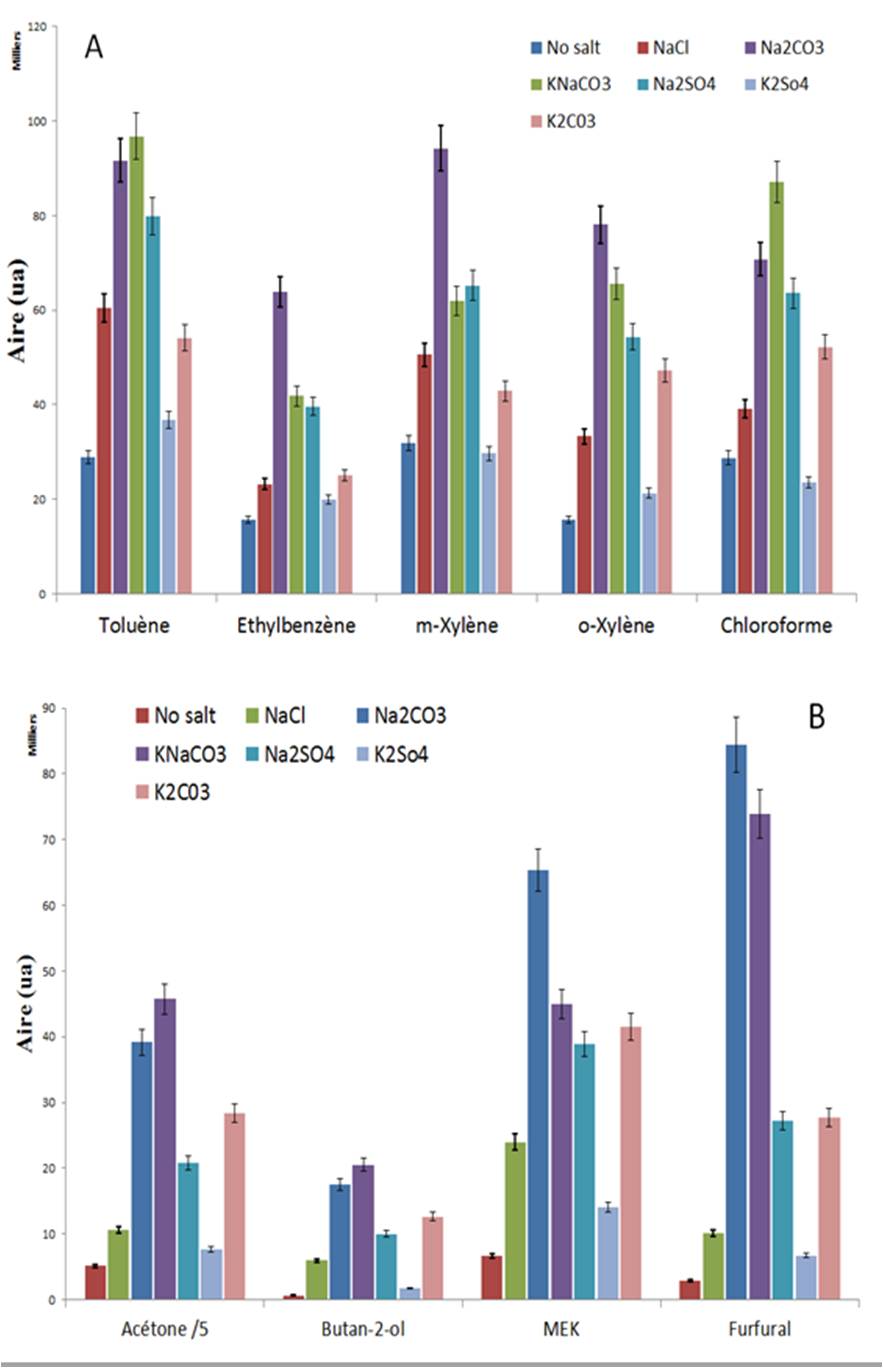
Influence of four grams of salts on gas concentration for each compound:
A) With apolar analyte
B) With polar analyte
|



