|
Introduction
Recently, the reaction pathway of pyridine and its derivative, particularly pyridinium ions, has been increasingly interesting as a model reaction of biologically important compounds such as nicotinamide. Few attempts were made to identify reaction products and these may depend upon the electrolytic conditions such as solvents. The cathodic and the anodic reductions of pyridinium ion were studied a few years ago however, the electrochemistry of pyridine has not yet been established.
Generally, the pyridine cathodic reaction has been observed in aqueous solutions. But the LMI, Laboratory of Multimaterials and Interfaces, investigated the anodic reduction of pyridine in acetonitrile.
Experimental conditions
Experiments were realized at room temperature under a nitrogen atmosphere. Cyclic voltammetry was performed using a potentiostat. The working electrode used was a Platinum disc (area: 2x10-3 cm²) and a platinum wire was used as the counter electrode. The reference electrode was an Ag/AgClO4 (0.1 M) couple in Acetonitrile.
The electrodes were put in the solvent, in which pyridine (2.5 mM) was added.
Results
Figure 1 shows the cyclic voltammogram of pyridine on a platinum electrode in acetonitrile solution. The first wave, which has a peak potential Ep of -1.07 V, is caused by the reduction of pyridine into a pyridinium ion. The second wave, which appeared at a potential peak of -0.65 V, is caused by the oxidation of the pyridinium ion.
When pyridine was added to the solution, the first wave increased. This means that peak height is proportional to the quantity of pyridine added. Figure 1 shows the evolution of peak current depending on pyridine concentration in the electrolytic solution.
Conclusion
The results of this work show that pyridine reduction can be observed in a non-aqueous solvent, such as acetonitrile. Moreover, the cyclic voltammetry of pyridine is reversible and is a non destructive method.
|
|
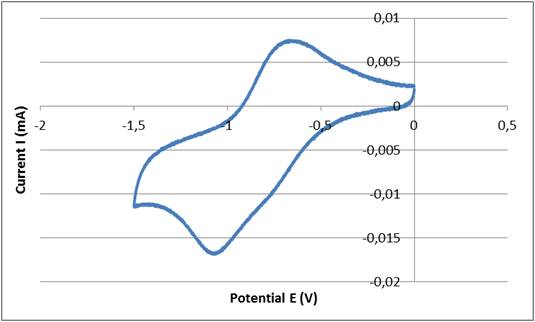
Cyclic voltammogram of pyridine
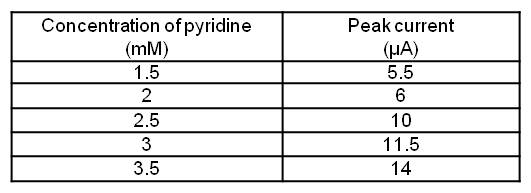
Peak current depending on pyridine concentration
|




