|
Introduction
Workplace sample is necessary to prevent chemical risks to which employees are exposed daily. Among the numerous pollutants: Sodium and Potassium Hydroxide. Both are part of the prevention of the occupational risks because they can be found in smokes in surface treatment facilities. The inhalation of these two compounds can provoke the irritation of the respiratory tract.
NaOH and KOH quantitative methods using titration and capillary electrophoresis had been developed by the French National Research and Safety Institute for the Prevention of Occupational accidents and Diseases. However, the first one is not selective and the other one is not available in the laboratory. That is why an alternative is necessary: IC. It quantifies compounds via their ion (Na+ and K+).
This article deals with the establishment of the experimental procedure. Besides, the rightness of the method is checked by comparison of atmospheric sample quantification by titration and IC.
Experimental conditions
Atmospheric particles were collected by a cassette on which a Polytetrafluoroethylen filter was placed. On the sampling site, the cassette was connected to a sampling pump by means of a tube.
Regarding the sample treatment, solvent desorption needed to be chosen. It is also used as standard making solvent. So it had to provide a good stability of the calibration curve. That is why, standard curve controls were made. Their calculated concentrations had to be between ±10% of the target value. The first attempt was to use Isopropyl alcohol (the one described in the titration method). Though, calibration curve stability was unsatisfying. All controls were out of the specification. The second attempt was to use ultrapure water as solvent. Controls were in the specification. Consequently, the particles collected by the filter were desorbed in 10mL of ultrapure water.
Then, as requested in the titration method, it underwent a step of sonication for 10 minutes. Afterward, the solution was filtered with Polyester-extra filter.
It was finally possible to analyze the sample by IC. Bibliographic study allows us to find the relevant parameters to apply to the chromatographic system:
Stationary phase: CS16 column (3x250 mm) – Mobil phase: Methansulfonic acid 30 mM – Flow: 0.5 mL/min – Conductivity detector (ICS-300 DC) with ion suppression (CSRS ULTRA 2 mm) – Suppressor current: 50 mA – Cell Heater temperature: 35 °C – Column temperature: 30 °C – Detector compartment temperature: 30 °C.
Results
In order to verify the method rightness, two atmospheric samples were done simultaneously on the same workstation. One was analyzed by IC and the other one by titration.
According to the IC analysis, only NaOH is present in samples. NaOH concentration results of the external calibration are summarized on the data table.
Titration and IC results are concordant for the high concentrated samples (variation rate < 15%). Nonetheless, for the lows, the variation rate is important (up to 130%). Without further experiments, the difference origins are difficult to explain. However, hypotheses can be made. For example, this can be explained by the non homogeneity of the samples.
Conclusion
Techniques, experimental parameters, reagents … had to be carefully chosen. All in all, they allow us to answer the issue.
|
|
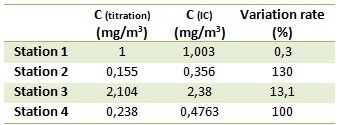
Data table: Calculated concentration of sample analyzed by titration and IC.
|


