|
Introduction
La Drome laboratoire is located near Valence and its main objective is to analyses numerous samples for companies or private individuals.
Different concentrations of metal in matrix such as sediment, mud or sand were determined. For the mineralization process, hydrofluoric acid was used whereas most laboratories do not. This is because this acid is very hazardous; its burns can result in amputation in the worst case because F- ion precipitates with Ca 2+ in the living cells and it kills them. Moreover, the burn is initially painless. This acid is one of the few chemicals known to dissolve glass.
But it is way more efficient than nitric acid for dissolving solid samples that is La Drome laboratoire is still using it.
Experimental conditions
0.1250 g of the MRC PACS-1 was mineralized in 9 different tubes. In the first three, 1260 µL of hydrochloric acid 35% was added, in the next three 932 µL of nitric acid 69% and in the last three 500 µL of hydrofluoric acid 50%. That way, there was 1.45.10-2 moles of acid per tube then the tubes were left for 12 hours.
The next morning, 8 mL of distilled water and 2.5 mL of nitric acid were added in each tube. Next, all the tubes were micro-waved at 250 °C to finalize the mineralization. Subsequently, each tube was poured in a digitube and adjusted at 25 mL with distilled water. Then, every solution was diluted 10 times and 200 times to be sure that even the highest concentration of metal would be included in the game of calibration. The exact mass of PACS-1 and the dilution factor for each sample was entered in the ICP-AES software to give the concentration in mg/kg directly.
Results
Calcium, cobalt and antimony were chosen. The concentration of each element in the MRC was 19 g/kg for the calcium, 12.1 mg/kg for the cobalt and 14.6 mg/kg for the antimony. The results obtained with each acid were compiled in the table 1.
Conclusion
We can see that the HF is the best for the mineralization followed by the HCl and the HNO3. HCl is a stronger acid than HNO3 (pKa(HCl) = -6.30 ; pKa(HNO3) = -1.8) but if we look only at the pKa, HF is supposed to be the weakest acid because its pKa is 3.20. This is because F- is a very small ion, which means that the charge is very localized so the reaction HF->H+ + F- is not very favorable.
|
|
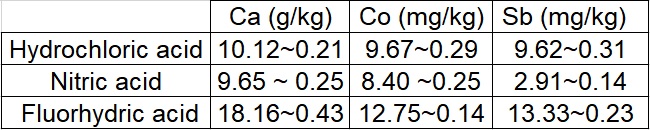
Table 1 Result obtained for each acid
|



