|
Introduction
Monolithic columns have been created 40 years ago and were designed to replace packed columns. Being easier to produce and resistant to high temperature and pressure, they have been extensively studied in gas chromatography (GC). However, after the introduction of open tubular capillary columns, currently the most used, monolithic columns were dropped. Since the 1990s, monolithic columns have returned mainly for LC separation but they are also of interest for their particular properties in high pressure gas chromatography.
Such columns have been investigated by GC. First, verification of system performances was conducted, and the system was optimized in order to study various monolithic columns.
In this article, the influence of the nature of the carrier gas on dispersion and retention are reported.
Experimental conditions
Agilent 7820A gas chromatograph was modified by SRA Instruments: gas sample was injected thanks to a 4-port Valco valve of 60 nL, supporting up to 69 bar. The detector was a flame ionization detector (FID) and Agilent Chemstation software was used to control the device, for data acquisition and for data processing. Two gas cylinders were used: one containing methane and ethane as studied sample, and a second cylinder containing helium or nitrogen was used as carrier gas.
The capillary column used is a silica based monolith called L2C3 by privacy concerns, it measures 63,5 cm in length, 70 µm in diameter with pores diameters of about 2,2 µm.
Results
Fig 1 shows that the minimum plate height (Hmin) using nitrogen as carrier gas is lower that when using helium. However, the optimum velocity (uopt) is lower in the case of nitrogen than in helium.
Fig 2 shows the logarithm of the retention factor (k) of ethane as a function of the carrier gas inlet pressure. Indeed, using helium, the logarithm of the retention factor k for a given temperature remains constant as expected for GC. However when nitrogen was used as carrier gas, the retention factor of ethane decreases when the carrier gas pressure increases suggesting interactions between mobile phase and stationary phase, or between ethane and mobile phase, as observed in LC and SFC.
Conclusion
In contrast to gas chromatography with open tubular columns used at low pressure (less than 3 bar), in high pressure gas chromatography using monolithic capillary column based on silica, the carrier gas cannot be considered as an inert mobile phase. Depending on the nature of the carrier gas, its pressure can influence the retention of ethane.
|
|
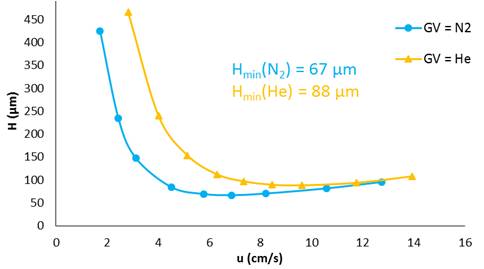
Van Deemter curve of ethane with nitrogen and helium used as carrier gas.
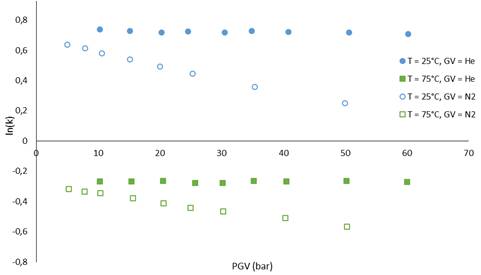
Influence of nature and carrier gas pressure on the retention factor k of ethane at 25 and 75°C.
|




