|
Introduction
Nowadays, industries reject a lot of pollutants like CO2 or ammonia. The ammonia needs to be treated because it can lead to health issues and difficulties in the treatment of water via chloration. We are looking for a way to treat ammonia that releases non-pollutants compounds like N2. In order to do that, we oxidize it with air under high pressure and high temperature. The goal of this internship was to elaborate a retention model to separate and quantify ammonia and N2 so as to check the entire transformation of ammonia into N2 with a Gas Chromatograph (GC).
Experimental conditions
The GC is composed of 3 columns: the first one is for the separation of water and carbonated compounds, the second one is for the separation of water and ammonia and the last one for the separation of the permanent gases (O2, He, N2) (composed of a mole sieve of 5 A). As the last column cannot stand water, it is avoided during analysis thanks to a valve system. The vector gas is hydrogen and the GC is directly connected to the tank where we do the catalyzed reaction of ammonia into N2.
Results
We take a gas flow of 7 mL/min.
To analyze ammonia and N2, we needed to do two retention models: one with the separation of water, ammonia and permanent gazes, and the other one for the separation of permanent gazes.
In the graph below, we can see that whatever the temperature, each compound is separated from the others. So, we took a higher temperature to have a good separation and a fast analysis.
Conclusion
To conclude, we saw that the retention of our compounds was function of the temperature of the oven of the GC. And so, we saw that the optimal temperatures for the analysis for these compounds are 110°C in a first time and 100°C in a second time.
|
|
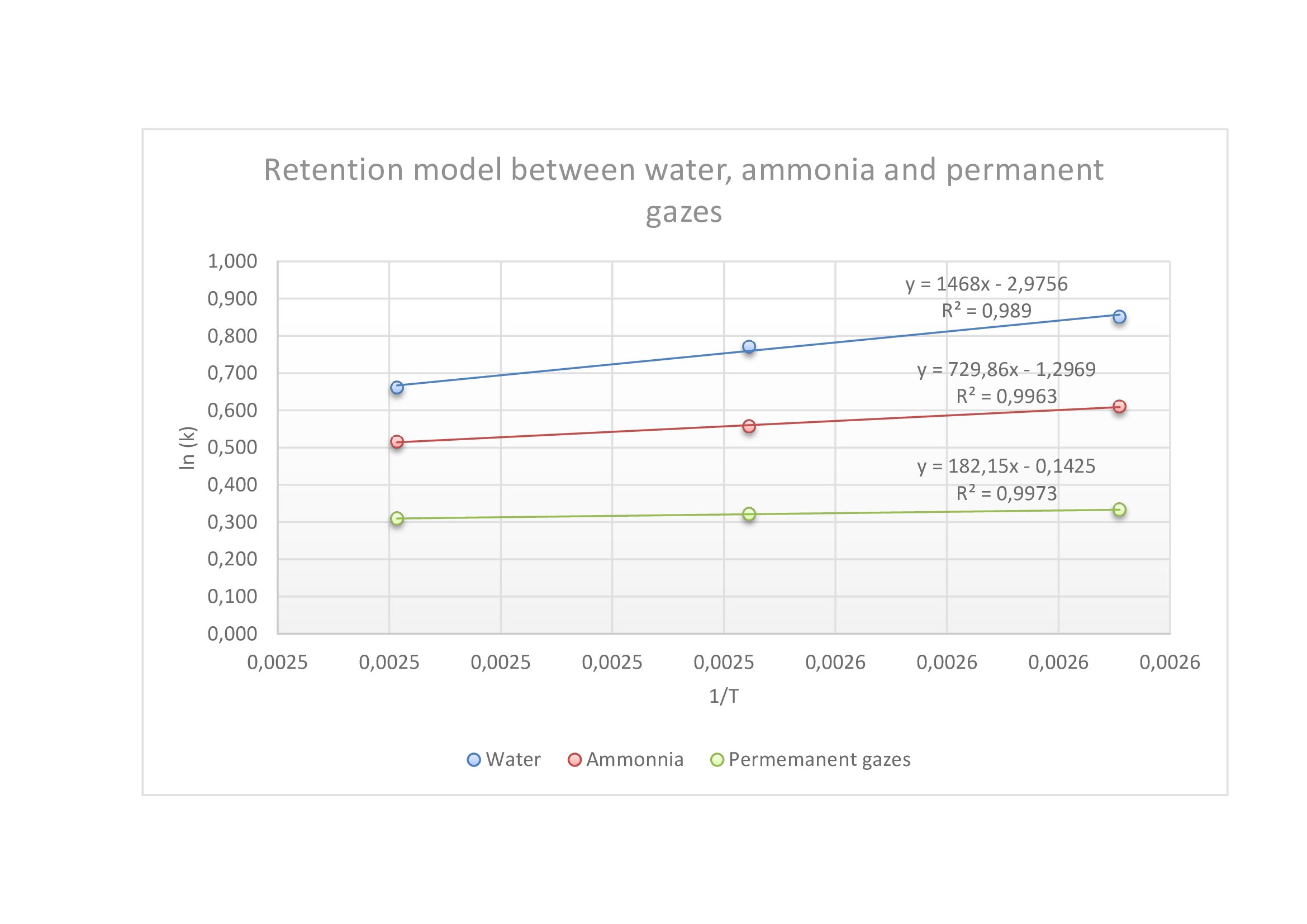
Retention model between ammonia, water and permanent gazes (flow : 7mL/min)
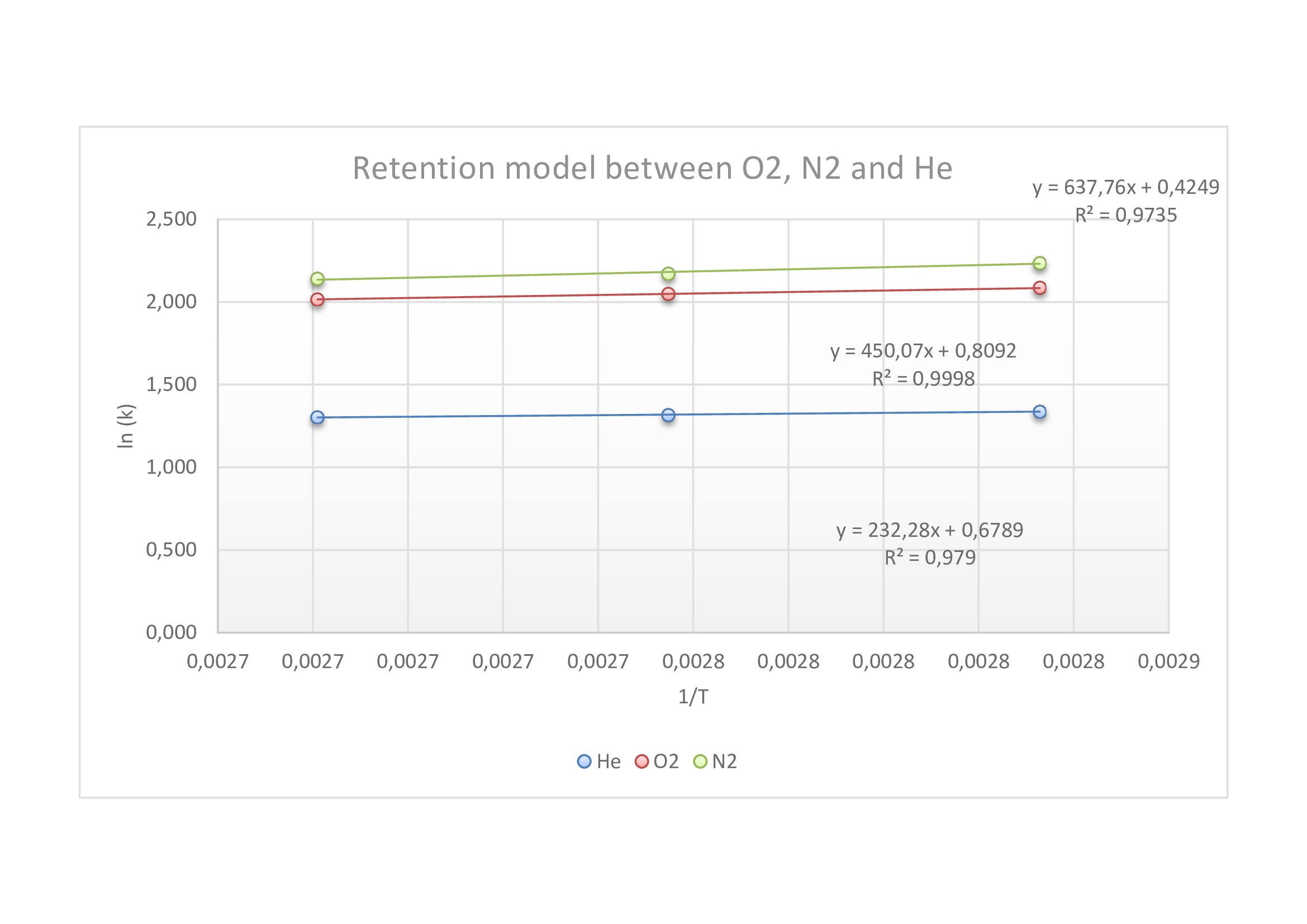
Retention model between O2, N2 and He (flow : 7mL/min)
|




